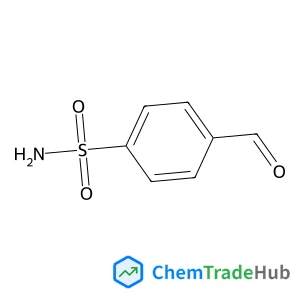
4-Formylbenzenesulfonamide | CAS No. 3240-35-5
Basic Information
CAS Number
3240-35-5
Molecular Formula
C7H7NO3S
Molecular Weight
185.20 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
392.7℃ at 760 mmHg
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Class
IRRITANT
Synonyms & References
English
- UNII-8H09AK2C6C
- 4-formylbenzenesulfonamide
- PCPUKVSTMLHXQF-UHFFFAOYSA-N
- 4-formyl-benzenesulfonamide
- 4-aminosulfonylbenzaldehyde
- Benzenesulfonamide, 4-formyl-
- ZB0762
- p-Aminosulphonylbenzaldehyde
- A821262
- 3240-35-5
- Q27270452
- AKOS005172423
- BB 0259244
- SY082574
- p-aminosulfonylbenzaldehyde
- P-FORMYLBENZENESULFONAMIDE
- 4-(formyl)benzenesulfonamide
- FT-0654328
- p-Sulfamoylbenzaldehyd
- Benzenesulfonamide, p-formyl- (7CI,8CI); 4-Formylbenzenesulfonamide; 4-Sulfamoylbenzaldehyde; p-(Aminosulfonyl)benzaldehyde; p-Formylbenzenesulfonamide
- MFCD03196508
- DTXSID80186114
- Z1198161671
- SCHEMBL663729
- 4-Desaminomethyl 4-Formylmafenide
- 8H09AK2C6C
- 4-formylbenzene-1-sulfonamide
- p-Sulfamoylbenzaldehyde
- Benzenesulfonamide, 4-formyl- (9CI)
- P-(AMINOSULFONYL)BENZALDEHYDE
- AS-38855
- CS-0012134
- 4-Sulfamoylbenzaldehyde
- AMY27456
- EN300-199160
- 4-Formylbenzenesulfonamide
- 4-Formylbenzenesulfonamide
- Benzenesulfonamide,4-formyl-
- 4-formylbenzene-sulfonamide
- 4-methanoylbenzenesulfonamide
- Benzenesulfonamide,4-formyl
MDL_Number
MFCD03196508
CAS Number
3240-35-5
Customs_Code
2935009090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Tengzhun BioScience Co., Ltd. China - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 China - Shanghai Yuyeh Biotechnology Co., Ltd. China - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 China - Guangzhou Xufan Trading Co., Ltd. China - Guangzhou Xufan Trading Co., Ltd. |
|||||
 China - Shandong Academy of Pharmaceutical Sciences Pilot Plant China - Shandong Academy of Pharmaceutical Sciences Pilot Plant |
|||||
 China - Shandong Boxing Xinhuang Food Machinery Industry Co., Ltd. China - Shandong Boxing Xinhuang Food Machinery Industry Co., Ltd. |
Related Compounds
Related Articles
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J