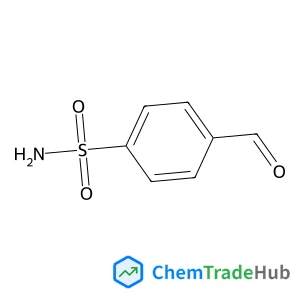
4-Formylbenzenesulfonamide(CAS号:3240-35-5)
4-甲酰基苯磺酰胺
基本信息
CAS号
3240-35-5
分子式
C7H7NO3S
分子量
185.20 g/mol
Quick Actions
基本物理性质
沸点
392.7℃ at 760 mmHg
安全信息
查看安全信息危险类别
IRRITANT
同义词与参考文献
英文
- UNII-8H09AK2C6C
- 4-formylbenzenesulfonamide
- PCPUKVSTMLHXQF-UHFFFAOYSA-N
- 4-formyl-benzenesulfonamide
- 4-aminosulfonylbenzaldehyde
- Benzenesulfonamide, 4-formyl-
- ZB0762
- p-Aminosulphonylbenzaldehyde
- A821262
- 3240-35-5
- Q27270452
- AKOS005172423
- BB 0259244
- SY082574
- p-aminosulfonylbenzaldehyde
- P-FORMYLBENZENESULFONAMIDE
- 4-(formyl)benzenesulfonamide
- FT-0654328
- p-Sulfamoylbenzaldehyd
- Benzenesulfonamide, p-formyl- (7CI,8CI); 4-Formylbenzenesulfonamide; 4-Sulfamoylbenzaldehyde; p-(Aminosulfonyl)benzaldehyde; p-Formylbenzenesulfonamide
- MFCD03196508
- DTXSID80186114
- Z1198161671
- SCHEMBL663729
- 4-Desaminomethyl 4-Formylmafenide
- 8H09AK2C6C
- 4-formylbenzene-1-sulfonamide
- p-Sulfamoylbenzaldehyde
- Benzenesulfonamide, 4-formyl- (9CI)
- P-(AMINOSULFONYL)BENZALDEHYDE
- AS-38855
- CS-0012134
- 4-Sulfamoylbenzaldehyde
- AMY27456
- EN300-199160
- 4-Formylbenzenesulfonamide
- 4-Formylbenzenesulfonamide
- Benzenesulfonamide,4-formyl-
- 4-formylbenzene-sulfonamide
- 4-methanoylbenzenesulfonamide
- Benzenesulfonamide,4-formyl
中文
- 4-甲酰苯磺酰胺
- 4-甲酰基苯磺酰胺
MDL_Number
MFCD03196508
CAS号
3240-35-5
Customs_Code
2935009090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 深圳方寸达科技有限公司 中国 - 深圳方寸达科技有限公司 |
|||||
 中国 - 昱奎化工有限责任公司 中国 - 昱奎化工有限责任公司 |
|||||
 中国 - 北京环宇京辉京城气体科技有限公司 中国 - 北京环宇京辉京城气体科技有限公司 |
相关文献
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B