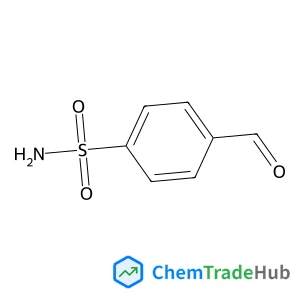
4-Formylbenzenesulfonamide(CAS番号:3240-35-5)
基本情報
CAS番号
3240-35-5
分子式
C7H7NO3S
分子量
185.20 g/mol
Quick Actions
基本的な物理特性
沸点
392.7℃ at 760 mmHg
安全情報
安全情報を表示危険物区分
IRRITANT
同義語と参考文献
英語
- UNII-8H09AK2C6C
- 4-formylbenzenesulfonamide
- PCPUKVSTMLHXQF-UHFFFAOYSA-N
- 4-formyl-benzenesulfonamide
- 4-aminosulfonylbenzaldehyde
- Benzenesulfonamide, 4-formyl-
- ZB0762
- p-Aminosulphonylbenzaldehyde
- A821262
- 3240-35-5
- Q27270452
- AKOS005172423
- BB 0259244
- SY082574
- p-aminosulfonylbenzaldehyde
- P-FORMYLBENZENESULFONAMIDE
- 4-(formyl)benzenesulfonamide
- FT-0654328
- p-Sulfamoylbenzaldehyd
- Benzenesulfonamide, p-formyl- (7CI,8CI); 4-Formylbenzenesulfonamide; 4-Sulfamoylbenzaldehyde; p-(Aminosulfonyl)benzaldehyde; p-Formylbenzenesulfonamide
- MFCD03196508
- DTXSID80186114
- Z1198161671
- SCHEMBL663729
- 4-Desaminomethyl 4-Formylmafenide
- 8H09AK2C6C
- 4-formylbenzene-1-sulfonamide
- p-Sulfamoylbenzaldehyde
- Benzenesulfonamide, 4-formyl- (9CI)
- P-(AMINOSULFONYL)BENZALDEHYDE
- AS-38855
- CS-0012134
- 4-Sulfamoylbenzaldehyde
- AMY27456
- EN300-199160
- 4-Formylbenzenesulfonamide
- 4-Formylbenzenesulfonamide
- Benzenesulfonamide,4-formyl-
- 4-formylbenzene-sulfonamide
- 4-methanoylbenzenesulfonamide
- Benzenesulfonamide,4-formyl
MDL_Number
MFCD03196508
CAS番号
3240-35-5
Customs_Code
2935009090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 中国 - Zoucheng Tianxing Chemical Co., Ltd. 中国 - Zoucheng Tianxing Chemical Co., Ltd. |
|||||
 メキシコ - Alkem de México, S.A. de C.V. メキシコ - Alkem de México, S.A. de C.V. |
|||||
 ドイツ - NETZSCH-Feinmahltechnik GmbH ドイツ - NETZSCH-Feinmahltechnik GmbH |
関連論文
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementation
Akira Takai, Keiko Yoshizawa
DOI: 10.1039/C9CC08664A

![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)
![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)