C3-Functionalization of indoles with α-heteroaryl-substituted methyl alcohols
Literature Information
Ethan J. Pazur, Nikhil R. Tasker, Peter Wipf
The transition metal-free Cs2CO3/Oxone®-mediated C3-alkylation of indoles proceeds in moderate to high yields with a variety of C4–C7 functionalized indoles and is applicable to 2-, 3- and 4-hydroxymethyl pyridines and related electron-deficient heterocycles, permitting novel late-stage drug functionalizations. Preliminary mechanistic studies support a hydrogen autotransfer-type chain process starting with an initial oxidation of the alcohol to the corresponding aldehyde, followed by a subsequent condensation onto indole and reduction/hydride delivery from another equivalent of the primary alcohol.
Related Literature
IF 4.616
A compact high resolution ion mobility spectrometer for fast trace gas analysisIF 4.616
Inside front coverIF 4.616
Comparison of aggregating agents for the surface-enhanced Raman analysis of benzodiazepinesIF 4.616
Fourier transform infrared imaging analysis in discrimination studies of bladder cancerIF 4.616
Melamine modified gold nanoprobe for “on-spot” colorimetric recognition of clonazepam from biological specimensIF 4.616
Multicolour probes for sequence-specific DNA detection based on graphene oxideIF 4.616
A protein nanofiber hydrogel for sensitive immunoassaysIF 4.616
A molecularly-imprinted electrochemical sensor based on a graphene–Prussian blue composite-modified glassy carbon electrode for the detection of butylated hydroxyanisole in foodstuffsIF 4.616
High-throughput analysis of drugs in biological fluids by desorption electrospray ionizationmass spectrometry coupled with thin liquid membrane extractionIF 4.616
Source Journal
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
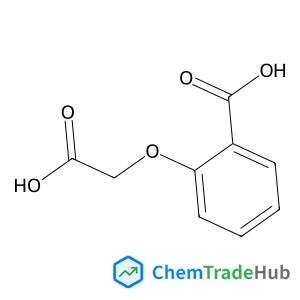
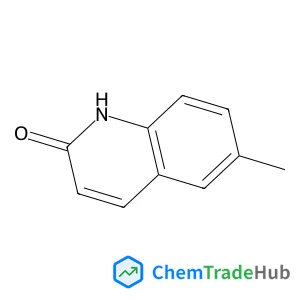
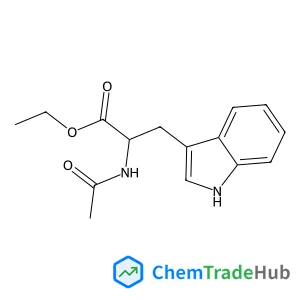
Recommended Compounds
Recommended Suppliers
 Riddell Biotechnology (Shanghai) Co., Ltd.
Riddell Biotechnology (Shanghai) Co., Ltd. Zibo Shengshi Da Chemical Technology Co., Ltd
Zibo Shengshi Da Chemical Technology Co., Ltd Xingpeng Compound Fertilizer Co., Ltd., Dashaqiao City
Xingpeng Compound Fertilizer Co., Ltd., Dashaqiao City ita-Tech GmbH
ita-Tech GmbH Linghai City Chen Guang Pharmaceutical Chemical Factory
Linghai City Chen Guang Pharmaceutical Chemical Factory Profimess GmbH
Profimess GmbH Advan pharmachem co., ltd
Advan pharmachem co., ltd Goudsche Machinefabriek B.V. (GMF-Gouda BV)
Goudsche Machinefabriek B.V. (GMF-Gouda BV) Xinxiang BaoLin Vibration Sieving Equipment Factory
Xinxiang BaoLin Vibration Sieving Equipment Factory Incoatec GmbH
Incoatec GmbH









![175202-29-6 - 2-[1-Methyl-3-(trifluoromethyl)pyrazol-5-yl]thiophene-5-carboxylic acid 175202-29-6 - 2-[1-Methyl-3-(trifluoromethyl)pyrazol-5-yl]thiophene-5-carboxylic acid](/structs/175/175202-29-6-812a.webp)