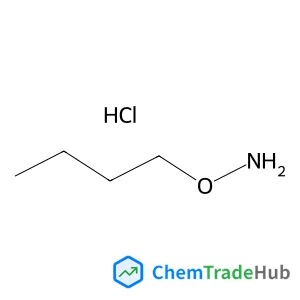
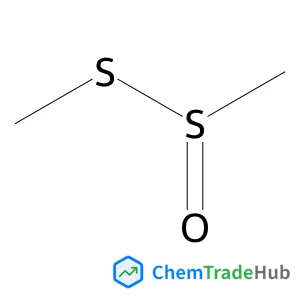
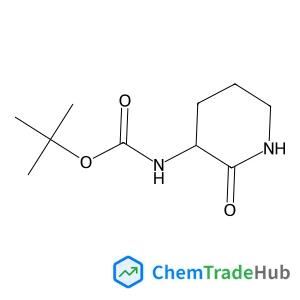
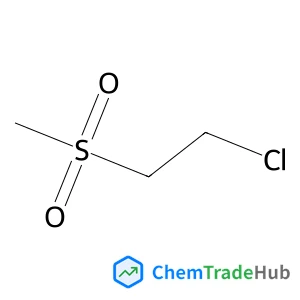
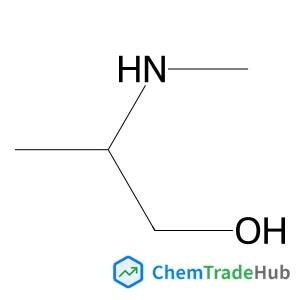
C3-Functionalization of indoles with α-heteroaryl-substituted methyl alcohols
文献信息
Ethan J. Pazur, Nikhil R. Tasker, Peter Wipf
The transition metal-free Cs2CO3/Oxone®-mediated C3-alkylation of indoles proceeds in moderate to high yields with a variety of C4–C7 functionalized indoles and is applicable to 2-, 3- and 4-hydroxymethyl pyridines and related electron-deficient heterocycles, permitting novel late-stage drug functionalizations. Preliminary mechanistic studies support a hydrogen autotransfer-type chain process starting with an initial oxidation of the alcohol to the corresponding aldehyde, followed by a subsequent condensation onto indole and reduction/hydride delivery from another equivalent of the primary alcohol.
相关文献
IF 6.222
A model-based comparison of Ru and Ni catalysts for the Sabatier reactionIF 6.367
Front coverIF 6.843
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡IF 6.222
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countriesIF 6.367
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis processIF 6.367
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular deviceIF 6.367
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)IF 6.222
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon clothIF 6.367
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteriesIF 6.222
来源期刊
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
推荐供应商
 广州市耿达贸易有限公司
广州市耿达贸易有限公司 郑州联创食化工贸有限公司
郑州联创食化工贸有限公司 技术进步Dieter Verhees
技术进步Dieter Verhees 斯百全化学(上海)有限公司
斯百全化学(上海)有限公司 镇江市海通化工有限公司
镇江市海通化工有限公司 霍布雷仪器公司
霍布雷仪器公司 英塔斯科学成像仪器有限公司
英塔斯科学成像仪器有限公司 KIMAESA AG
KIMAESA AG 北京沃比森科技有限公司
北京沃比森科技有限公司 CARBOGEN AMCIS AG
CARBOGEN AMCIS AG