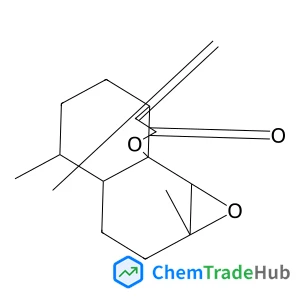
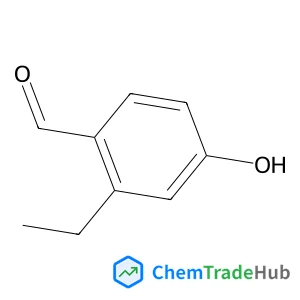
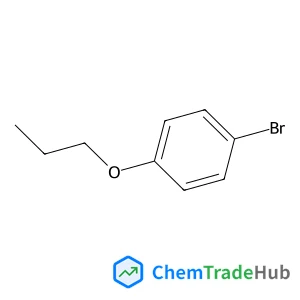
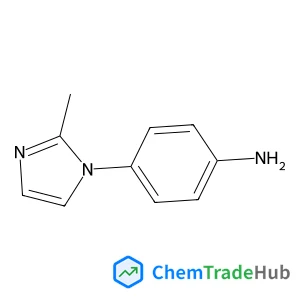
C3-Functionalization of indoles with α-heteroaryl-substituted methyl alcohols
文献情報
Ethan J. Pazur, Nikhil R. Tasker, Peter Wipf
The transition metal-free Cs2CO3/Oxone®-mediated C3-alkylation of indoles proceeds in moderate to high yields with a variety of C4–C7 functionalized indoles and is applicable to 2-, 3- and 4-hydroxymethyl pyridines and related electron-deficient heterocycles, permitting novel late-stage drug functionalizations. Preliminary mechanistic studies support a hydrogen autotransfer-type chain process starting with an initial oxidation of the alcohol to the corresponding aldehyde, followed by a subsequent condensation onto indole and reduction/hydride delivery from another equivalent of the primary alcohol.
関連文献
IF 6.222
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteriesIF 6.367
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursorIF 6.222
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA deliveryIF 6.843
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen sourceIF 6.367
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon clothIF 6.367
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)IF 6.222
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cellsIF 6.367
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammationIF 6.222
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generationIF 6.367
掲載誌
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
おすすめサプライヤー
 Van Aroma
Van Aroma 深圳康宇达発光材料有限公司
深圳康宇达発光材料有限公司 Depthseng Dengnu Longbang Xincai Gongsi
Depthseng Dengnu Longbang Xincai Gongsi 天津旅畅科技发展有限公司
天津旅畅科技发展有限公司 浙江华晟化学製品有限公司
浙江华晟化学製品有限公司 潍坊昌盛硝盐有限公司
潍坊昌盛硝盐有限公司 SITA Messtechnik GmbH
SITA Messtechnik GmbH ミシェル・バウレ SA
ミシェル・バウレ SA 浙江隆源フィルタープレス有限会社
浙江隆源フィルタープレス有限会社 MSR エレクトロニクス GmbH
MSR エレクトロニクス GmbH