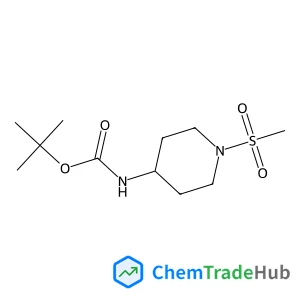
tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate | CAS No. 287953-38-2
Basic Information
CAS Number
287953-38-2
Molecular Formula
C11H22N2O4S
Molecular Weight
278.37 g/mol
Quick Actions
Basic Physical Properties
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H302-H315-H319-H335
Synonyms & References
English
- tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate
- tert-Butyl (1-(methylsulfonyl)-piperidin-4-yl)carbamate
- TERT-BUTYL 1-(METHYLSULFONYL)PIPERIDIN-4-YLCARBAMATE
- (1-methanesulfonyl-piperidin-4-yl)carbamic acid tert-butyl ester
- (1-methanesulfonyl-piperidin-4-yl)-carbamic acid tert-butyl ester
- 4-(N-BOC-amino)-1-methanesulphonylpiperidine
- ANW-73562
- CTK8C4939
- STK360719
- SureCN3841283
- t-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl N-(1-methanesulfonylpiperidin-4-yl)carbamate
- tert-butyl N-(1-methylsulfonylpiperidin-4-yl)carbamate
- BVSNHVUSSUREGZ-UHFFFAOYSA-N
- ST2407593
- t-butyl[1-(methylsulfonyl)pipe
- t-butyl[1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-Butyl [1-(methanesulfonyl)piperidin-4-yl]carbamate
- CS-0154402
- tert-Butyl 1-(methylsulfonyl)piperidin-4-ylcarbamate
- tert-Butyl(1-(methylsulfonyl)piperidin-4-yl)carbamate
- A876622
- C11H22N2O4S
- Carbamic acid, N-[1-(methylsulfonyl)-4-piperidinyl]-, 1,1-dimethylethyl ester
- SCHEMBL3841283
- (1-methanesulfonyl-piperidin4-yl)-carbamic acid tert-butyl ester
- DB-352712
- AKOS005441848
- C74477
- MFCD11852586
- DTXSID10649484
- SB43153
- DS-2678
- Z741374170
- 287953-38-2
MDL_Number
MFCD11852586
CAS Number
287953-38-2
Customs_Code
2935009090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. China - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 China - Shanghai Tengzhun BioScience Co., Ltd. China - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shanghai Jixiang Biotechnology Co., Ltd. China - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 China - Shijiazhuang Tianaoyue Fine Chemical Co., Ltd. China - Shijiazhuang Tianaoyue Fine Chemical Co., Ltd. |
|||||
 China - Zhejiang Weirong Pharmaceutical Chemical Co., Ltd. China - Zhejiang Weirong Pharmaceutical Chemical Co., Ltd. |
|||||
 Germany - Schramm Coatings GmbH Germany - Schramm Coatings GmbH |
Related Compounds
Related Articles
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H