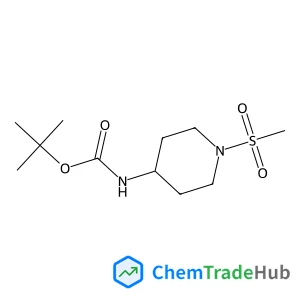
tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate(CAS番号:287953-38-2)
基本情報
CAS番号
287953-38-2
分子式
C11H22N2O4S
分子量
278.37 g/mol
Quick Actions
基本的な物理特性
安全情報
安全情報を表示危険性の表示
H302-H315-H319-H335
同義語と参考文献
英語
- tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate
- tert-Butyl (1-(methylsulfonyl)-piperidin-4-yl)carbamate
- TERT-BUTYL 1-(METHYLSULFONYL)PIPERIDIN-4-YLCARBAMATE
- (1-methanesulfonyl-piperidin-4-yl)carbamic acid tert-butyl ester
- (1-methanesulfonyl-piperidin-4-yl)-carbamic acid tert-butyl ester
- 4-(N-BOC-amino)-1-methanesulphonylpiperidine
- ANW-73562
- CTK8C4939
- STK360719
- SureCN3841283
- t-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl N-(1-methanesulfonylpiperidin-4-yl)carbamate
- tert-butyl N-(1-methylsulfonylpiperidin-4-yl)carbamate
- BVSNHVUSSUREGZ-UHFFFAOYSA-N
- ST2407593
- t-butyl[1-(methylsulfonyl)pipe
- t-butyl[1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-Butyl [1-(methanesulfonyl)piperidin-4-yl]carbamate
- CS-0154402
- tert-Butyl 1-(methylsulfonyl)piperidin-4-ylcarbamate
- tert-Butyl(1-(methylsulfonyl)piperidin-4-yl)carbamate
- A876622
- C11H22N2O4S
- Carbamic acid, N-[1-(methylsulfonyl)-4-piperidinyl]-, 1,1-dimethylethyl ester
- SCHEMBL3841283
- (1-methanesulfonyl-piperidin4-yl)-carbamic acid tert-butyl ester
- DB-352712
- AKOS005441848
- C74477
- MFCD11852586
- DTXSID10649484
- SB43153
- DS-2678
- Z741374170
- 287953-38-2
MDL_Number
MFCD11852586
CAS番号
287953-38-2
Customs_Code
2935009090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 ドイツ - SL Kunststofftechnik GmbH ドイツ - SL Kunststofftechnik GmbH |
|||||
 ドイツ - biotronix GmbH ドイツ - biotronix GmbH |
|||||
 中国 - Yingyang Chemical Purification Materials Factory, Changge City, Henan Province 中国 - Yingyang Chemical Purification Materials Factory, Changge City, Henan Province |
関連論文
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B

![56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol 56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol](/structs/568/56843-76-6-0035.webp)