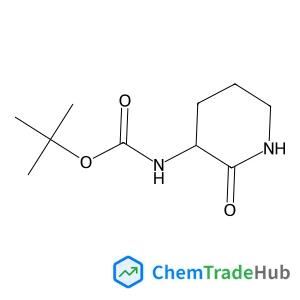
tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate(CAS号:287953-38-2)
(1-(甲基磺酰基)哌啶-4-基)氨基甲酸叔丁酯
基本信息
CAS号
287953-38-2
分子式
C11H22N2O4S
分子量
278.37 g/mol
Quick Actions
基本物理性质
安全信息
查看安全信息危险说明
H302-H315-H319-H335
同义词与参考文献
英文
- tert-Butyl (1-(methylsulfonyl)piperidin-4-yl)carbamate
- tert-Butyl (1-(methylsulfonyl)-piperidin-4-yl)carbamate
- TERT-BUTYL 1-(METHYLSULFONYL)PIPERIDIN-4-YLCARBAMATE
- (1-methanesulfonyl-piperidin-4-yl)carbamic acid tert-butyl ester
- (1-methanesulfonyl-piperidin-4-yl)-carbamic acid tert-butyl ester
- 4-(N-BOC-amino)-1-methanesulphonylpiperidine
- ANW-73562
- CTK8C4939
- STK360719
- SureCN3841283
- t-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl [1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-butyl N-(1-methanesulfonylpiperidin-4-yl)carbamate
- tert-butyl N-(1-methylsulfonylpiperidin-4-yl)carbamate
- BVSNHVUSSUREGZ-UHFFFAOYSA-N
- ST2407593
- t-butyl[1-(methylsulfonyl)pipe
- t-butyl[1-(methylsulfonyl)piperidin-4-yl]carbamate
- tert-Butyl [1-(methanesulfonyl)piperidin-4-yl]carbamate
- CS-0154402
- tert-Butyl 1-(methylsulfonyl)piperidin-4-ylcarbamate
- tert-Butyl(1-(methylsulfonyl)piperidin-4-yl)carbamate
- A876622
- C11H22N2O4S
- Carbamic acid, N-[1-(methylsulfonyl)-4-piperidinyl]-, 1,1-dimethylethyl ester
- SCHEMBL3841283
- (1-methanesulfonyl-piperidin4-yl)-carbamic acid tert-butyl ester
- DB-352712
- AKOS005441848
- C74477
- MFCD11852586
- DTXSID10649484
- SB43153
- DS-2678
- Z741374170
- 287953-38-2
中文
- 1-MS-4-Boc-氨基哌啶
- (1-(甲基磺酰基)哌啶-4-基)氨基甲酸叔丁酯
- [1-(甲磺酰基)哌啶-4-基]氨基甲酸叔丁酯
MDL_Number
MFCD11852586
CAS号
287953-38-2
Customs_Code
2935009090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 杭州药睿易成生物医药有限公司 中国 - 杭州药睿易成生物医药有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 金恩(广州)新材料有限公司 中国 - 金恩(广州)新材料有限公司 |
|||||
 印度 - 印度海湾公司 印度 - 印度海湾公司 |
|||||
 英国 - 阿尔法水分系统有限公司 英国 - 阿尔法水分系统有限公司 |
相关文献
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E