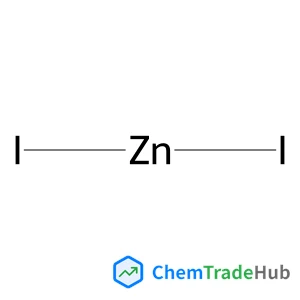
Zinc iodide | CAS No. 10139-47-6
Basic Information
CAS Number
10139-47-6
Molecular Formula
ZnI2
Molecular Weight
319.20 g/mol
Quick Actions
Basic Physical Properties
Melting Point
445 °C (lit.)
Boiling Point
624°C
Water Solubility
333 g/100 mL
Flash Point
625°C
Solubility
4500g/l
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H314
Hazard Class
8
Sensitiveness
Hygroscopic
Synonyms & References
English
- D04837
- EpiphaniQ SterileFor Oral INhalation Only
- EpiphaniQ
- CS-0015383
- NSC 39113
- 762R7A0O0B
- NSC-39113
- Zinc iodide, anhydrous, powder, 99.999% trace metals basis
- Zinc Iodide Powder
- Diiodozinc
- Zinc iodide, purum p.a., >=98.0% (AT)
- DTXSID5064968
- EINECS 233-396-0
- Zinc iodide
- 10139-47-6
- Zinc diiodide,99%
- Zinciodide
- Zinc iodide, >=98%
- Zinc iodide, anhydrous, beads, -10 mesh, 99.999% trace metals basis
- MFCD00011299
- EpiphaniQ Throat
- AKOS015951018
- Zinc iodide, >=99.99% trace metals basis
- J-000381
- Zinc iodide, ultra dry
- Zinc iodide (ZnI2)
- NSC39113
- UNII-762R7A0O0B
- ZINC IODIDE
- Hydriodic acid zinc salt (2:1)
- Zinc iodine
- ZnI2
- ZINC IODIDE PURE
- zinc iodide, ultra dry
- ZINCIODIDE,PURIFIED
- Zinc diiodide
- Zinc iodide anhydrous
- Zinc iodide(8CI)
- Zinc Iodid
- zinciodide(zni2)
- ZINC IODIDE, 98+%
MDL_Number
MFCD00011299
CAS Number
10139-47-6
Merck_Index
14,10140
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Sichuan Jupin Material Technology Co., Ltd. China - Sichuan Jupin Material Technology Co., Ltd. |
|||||
 China - Wuhan Xinzhike Chemical Technology Co., Ltd. China - Wuhan Xinzhike Chemical Technology Co., Ltd. |
|||||
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Hubei Shixing Chemical Co., Ltd. China - Hubei Shixing Chemical Co., Ltd. |
|||||
 China - Hubei Nona Technology Co., Ltd. China - Hubei Nona Technology Co., Ltd. |
|||||
 China - Youniu. com China - Youniu. com |
|||||
 Germany - Bureau Veritas Consumer Products Services Germany GmbH Germany - Bureau Veritas Consumer Products Services Germany GmbH |
|||||
 Austria - Bilfinger Industrietechnik Salzburg GmbH Austria - Bilfinger Industrietechnik Salzburg GmbH |
Related Compounds
Recommended Journals
Related Articles
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F