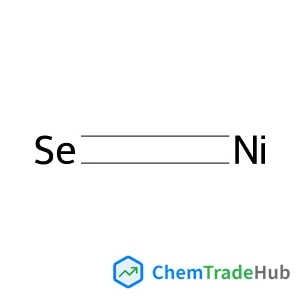
Selective and controllable amination and defluoroamidation of α-trifluoromethylstyrene
Literature Information
Shuang-Lian He, Yong-Sheng Bao, Juan Hu, Chaolumen Bai, Dan Liu
We present a blueprint for the amination and defluoroamidation of α-trifluoromethylstyrene. This practical protocol presents a general method for the diversity-oriented synthesis of vicinal trifluoromethyl amines and gem-difluoro alkenes from α-trifluoromethylstyrene maintaining excellent chemoselectivity. The synthetic strategy features outstanding atom economy and wide functional group tolerance under mild reaction conditions.
Related Literature
IF 4.616
Label-free electrochemical DNA sensing with a one-target-multitriggered hybridization chain reaction strategyIF 4.616
An X-ray transparent microfluidic platform for screening of the phase behavior of lipidic mesophasesIF 4.616
A biomimetic bitter receptor-based biosensor with high efficiency immobilization and purification using self-assembled aptamersIF 4.616
Dual signal amplification strategy for the fabrication of an ultrasensitive electrochemiluminescenct aptasensorIF 4.616
Inside front coverIF 4.616
Monitoring of cellular behaviors by microcavity array-based single-cell patterningIF 4.616
Multiplexed detection of microRNAs by tuning DNA-scaffolded silver nanoclustersIF 4.616
Colorimetric microchip assay using our own whole blood collected by a painless needle for home medical careIF 4.616
Direct comparison of fatty acid ratios in single cellular lipid droplets as determined by comparative Raman spectroscopy and gas chromatographyIF 4.616
Source Journal
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
Recommended Compounds
Recommended Suppliers
 Green Hydrogen Technology GmbH
Green Hydrogen Technology GmbH WenDa Chemical Co., Ltd. Ezhou City
WenDa Chemical Co., Ltd. Ezhou City Wuhai City Jinsu Humate Trading Co., Ltd.
Wuhai City Jinsu Humate Trading Co., Ltd. Zibo Shengshi Da Chemical Technology Co., Ltd
Zibo Shengshi Da Chemical Technology Co., Ltd Helm Skandinavien A/S
Helm Skandinavien A/S Zhonglian Chemical Products Co., Ltd. of Anshan City
Zhonglian Chemical Products Co., Ltd. of Anshan City TTS France Sàrl
TTS France Sàrl Química de Norteamérica, S. de R.L. de C.V. (Quiminort)
Química de Norteamérica, S. de R.L. de C.V. (Quiminort) awima engineering GmbH
awima engineering GmbH









![59669-26-0 - Dimethyl (1E,1'E)-N,N'-{sulfanediylbis[(methylcarbamoyl)oxy]}diethanimidothioate 59669-26-0 - Dimethyl (1E,1'E)-N,N'-{sulfanediylbis[(methylcarbamoyl)oxy]}diethanimidothioate](/structs/596/59669-26-0-4691.webp)