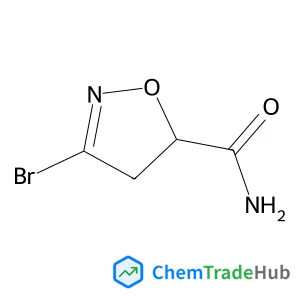
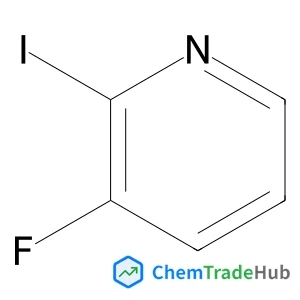
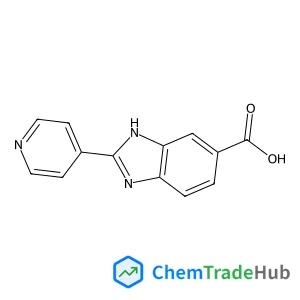
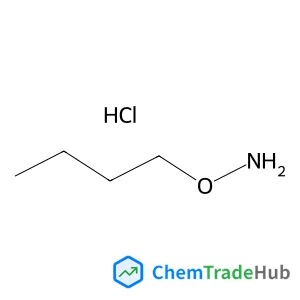
Selective and controllable amination and defluoroamidation of α-trifluoromethylstyrene
文献信息
Shuang-Lian He, Yong-Sheng Bao, Juan Hu, Chaolumen Bai, Dan Liu
We present a blueprint for the amination and defluoroamidation of α-trifluoromethylstyrene. This practical protocol presents a general method for the diversity-oriented synthesis of vicinal trifluoromethyl amines and gem-difluoro alkenes from α-trifluoromethylstyrene maintaining excellent chemoselectivity. The synthetic strategy features outstanding atom economy and wide functional group tolerance under mild reaction conditions.
相关文献
IF 6.367
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteriesIF 6.367
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursorIF 6.222
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteriesIF 6.367
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refineryIF 6.367
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cellsIF 6.367
Contents listIF 6.843
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayerIF 6.222
Contents listIF 6.222
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡IF 6.222
来源期刊
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
推荐供应商
 江苏万淇生物科技股份有限公司
江苏万淇生物科技股份有限公司 河南省长葛市明洋化工净化材料厂
河南省长葛市明洋化工净化材料厂 上海德祥医药技术有限公司
上海德祥医药技术有限公司 热系统有限公司KG
热系统有限公司KG 扬州长华生物科技有限公司
扬州长华生物科技有限公司 湖南省岳阳市云溪区道仁矶溶剂化
湖南省岳阳市云溪区道仁矶溶剂化 A-TEC工业集团
A-TEC工业集团 武汉荣申化工有限公司
武汉荣申化工有限公司 -。 威廉·施密特公司
-。 威廉·施密特公司 Loyal Gain International Enterprise Limited
Loyal Gain International Enterprise Limited