The highly enantioselective catalytic aza-Morita–Baylis–Hillman reaction
Literature Information
Fang-Le Hu, Min Shi
The highly enantioselective aza-Morita–Baylis–Hillman (aza-MBH) reaction is one of the most important reactions for the synthesis of optically active α-methylene-β-amino carbonyl compounds. The use of chiral phosphines or amines as organocatalysts can be envisaged for this catalytic asymmetric reaction. This mini review focuses on the important developments with regard to asymmetric aza-MBH reactions catalyzed by chiral phosphines or amines or even organometallic complexes in recent decades and also on the perspectives that these new developments offer.
Related Literature
IF 6.843
Inside back coverIF 6.222
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditionsIF 6.222
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular deviceIF 6.367
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementationIF 6.222
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structureIF 6.222
Milk exosomes with enhanced mucus penetrability for oral delivery of siRNAIF 6.843
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzerIF 6.367
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countriesIF 6.367
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)IF 6.222
Source Journal
Organic Chemistry Frontiers

Organic Chemistry Frontiers publishes high-quality research from across organic chemistry. Emphases are placed on studies that make significant contributions to the field of organic chemistry by reporting either new or significantly improved protocols or methodologies. Topics include, but are not limited to the following: Organic synthesis Development of synthetic methodologies Catalysis Natural products Functional organic materials Supramolecular and macromolecular chemistry Physical and computational organic chemistry
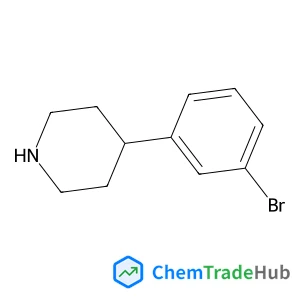
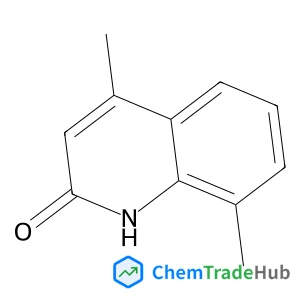
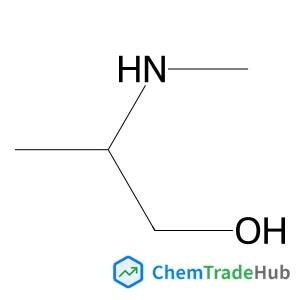
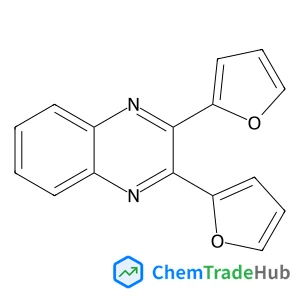
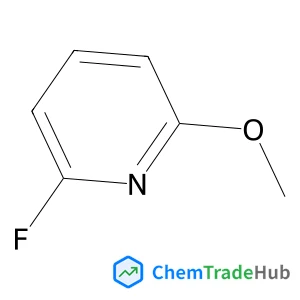
Recommended Compounds
Recommended Suppliers
 Jinhua Zhongneng Automatic Equipment Co., Ltd.
Jinhua Zhongneng Automatic Equipment Co., Ltd. Leyang Xingfa Manganese Industry Co., Ltd.
Leyang Xingfa Manganese Industry Co., Ltd. Romberger Maschinenfabrik GmbH
Romberger Maschinenfabrik GmbH Shaanxi Bolin Biotechnology Co., Ltd.
Shaanxi Bolin Biotechnology Co., Ltd. SITA Messtechnik GmbH
SITA Messtechnik GmbH Shanghai Youtuo Medicine Technology Co., Ltd.
Shanghai Youtuo Medicine Technology Co., Ltd. Zibo Dangao Chemical Industry Co., Ltd.
Zibo Dangao Chemical Industry Co., Ltd. Chromatographie-Zubehör Trott
Chromatographie-Zubehör Trott Printec S.A.
Printec S.A. Zhejiang Weirong Pharmaceutical Chemical Co., Ltd.
Zhejiang Weirong Pharmaceutical Chemical Co., Ltd.