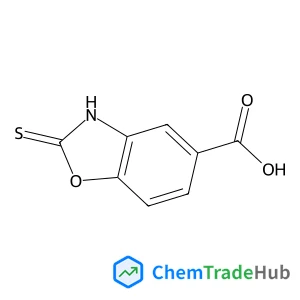
2-Thioxo-2,3-dihydro-1,3-benzoxazole-5-carboxylic acid | CAS No. 7341-98-2
Basic Information
CAS Number
7341-98-2
Molecular Formula
C8H5NO3S
Molecular Weight
195.20 g/mol
Quick Actions
Basic Physical Properties
Melting Point
280-283 ºC
Boiling Point
391 ºC
Density
1.66
Flash Point
191 ºC
Refractive Index
1.756
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- Oprea1_495052
- FT-0694100
- 2-sulfanyl-1,3-benzoxazole-5-carboxylic acid
- DTXSID00355946
- Z57478902
- AB10621
- 2-MERCAPTOBENZO[D]OXAZOLE-5-CARBOXYLIC ACID
- AG-205/14378040
- 2-MERCAPTOBENZOOXAZOLE-5-CARBOXYLICACID
- 2-MERCAPTO-1,3-BENZOXAZOLE-5-CARBOXYLIC ACID
- 7341-98-2
- MFCD01995748
- LS-07718
- F50795
- AKOS030229383
- SCHEMBL8653471
- 2-MERCAPTO-5-BENZOOXAZOLE-CARBOXYLIC ACID
- 2-sulfanylidene-3H-1,3-benzoxazole-5-carboxylic acid
- 2-mercaptobenzooxazole-5-carboxylic acid
- EN300-204255
- AKOS003272381
- A925808
- 2-MERCAPTOBENZO[D]OXAZOLE-5-CARBOXYLICACID
- 5-Benzoxazolecarboxylicacid, 2,3-dihydro-2-thioxo-
- 2-MERCAPTOBENZOOXAZOLE-5-CARBOXYLIC ACID
- 2-sulfanylbenzoxazole-5-carboxylic acid
- 2-Mercapto-5-benzoxazolecarboxylic acid
MDL_Number
MFCD01995748
CAS Number
7341-98-2
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 China - Shanghai Yuyeh Biotechnology Co., Ltd. China - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 China - Suzhou Shengjing Environmental Protection Equipment Co., Ltd China - Suzhou Shengjing Environmental Protection Equipment Co., Ltd |
|||||
 China - Shenzhen Kangyuda Luminescent Materials Co., Ltd. China - Shenzhen Kangyuda Luminescent Materials Co., Ltd. |
|||||
 Germany - DaXem GmbH Germany - DaXem GmbH |
|||||
 China - Quzhou Bangcheng Chemical Co., Ltd. China - Quzhou Bangcheng Chemical Co., Ltd. |
Related Compounds
Related Articles
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D

![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)
![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)









