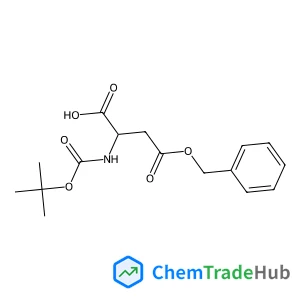
Boc-D-Asp(OBzl)-OH | CAS No. 51186-58-4
Basic Information
CAS Number
51186-58-4
Molecular Formula
C16H21NO6
Molecular Weight
323.34 g/mol
Quick Actions
Basic Physical Properties
Melting Point
101-105°C
Boiling Point
508.1℃ at 760 mmHg
Flash Point
261.1℃
Specific Rotation
+18° to +21° (c=1 in DMF)
Classification & Uses
Chemical Classification
Safety Information
Hazard Statement
H315; H319; H335
Hazard Class
IRRITANT
Synonyms & References
English
- MFCD00038255
- HY-W004083
- AC-30045
- Boc-D-aspartic acid b-benzyl ester
- Boc-D-Asp(OBzl)-OH, >=98.0% (HPLC)
- D-Aspartic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 4-(phenylmethyl) ester
- (R)-4-(Benzyloxy)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid
- AKOS015995303
- N-alpha-t-Butyloxycarbonyl-D-aspartic acid beta-benzyl ester
- Boc-D-aspartic acid 4-benzyl ester
- J-300057
- N-Boc-D-aspartic acid O-benzyl ester
- beta-Benzyl N-(tert-butoxycarbonyl)-D-aspartate
- CS-W004083
- JPD6M53HLN
- (2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxo-4-phenylmethoxybutanoic acid
- Q-101774
- (R)-4-(Benzyloxy)-2-((tert-butoxycarbonyl)amino]-4-oxobutanoic acid
- Boc-D-Asp(OBzl)-OH
- (R)-2-tert-butoxycarbonylamino-succinic acid 4-benzyl ester
- 51186-58-4
- (R)-4-(Benzyloxy)-2-(tert-butoxycarbonylamino)-4-oxobutanoic acid
- O10178
- SOHLZANWVLCPHK-GFCCVEGCSA-N
- Boc-D-aspartic acid ?-benzyl ester
- 4-(Phenylmethyl) hydrogen N-[(1,1-dimethylethoxy)carbonyl]-D-aspartate
- AS-19226
- Boc-D-Asp(OBn)-OH
- Boc-D-Aspartic acid 4-benzyl ester
- Boc-D-Asp(OBzl)-OHBoc-D-aspartic acid β-benzyl ester
- BOC-D-ASPARTIC ACID BENZYL ESTER
- Boc-D-aspartic acid β-benzyl ester
- Boc-D-Asp-OBzl
- N-Alpha-t-Boc-D-aspartic acid beta-benzyl ester
- BOC-D-Aspartic acid beta-benzyl ester
MDL_Number
MFCD00038255
CAS Number
51186-58-4
Customs_Code
2924299090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Yinxin Laboratory Equipment Co., Ltd. China - Shanghai Yinxin Laboratory Equipment Co., Ltd. |
|||||
 China - Beijing Bailingwei Technology Co., Ltd. China - Beijing Bailingwei Technology Co., Ltd. |
|||||
 China - Hubei Dahao Chemical Co., Ltd. China - Hubei Dahao Chemical Co., Ltd. |
|||||
 China - Hubei Shixing Chemical Co., Ltd. China - Hubei Shixing Chemical Co., Ltd. |
|||||
 China - Shanghai Yuyeh Biotechnology Co., Ltd. China - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 China - Guangzhou Xinxi Metallurgical Chemical Co., Ltd. China - Guangzhou Xinxi Metallurgical Chemical Co., Ltd. |
|||||
 China - Mota Xiamen Petroleum Technology Co., Ltd. China - Mota Xiamen Petroleum Technology Co., Ltd. |
|||||
 China - Xinxiang BaoLin Vibration Sieving Equipment Factory China - Xinxiang BaoLin Vibration Sieving Equipment Factory |
Related Compounds
Related Articles
Label-free electrochemical DNA sensing with a one-target-multitriggered hybridization chain reaction strategy
Zhu Zhu, Jianping Lei, Lin Liu, Huangxian Ju
DOI: 10.1039/C3AN01212C
Micro-analysis by near-infrared diffuse reflectance spectroscopy with chemometric methods
Yan Liu, Yu Ning, Wensheng Cai, Xueguang Shao
DOI: 10.1039/C3AN01232H
Dependence of the direct electron transfer activity and adsorption kinetics of cytochrome c on interfacial charge properties
Min Wang, Zeng-Qiang Wu, Wen-Jing Bao, Yue Zhou, Xing-Hua Xia
DOI: 10.1039/C3AN01042B
Dual signal amplification strategy for the fabrication of an ultrasensitive electrochemiluminescenct aptasensor
Min Zhao, Ying Zhuo, Yaqin Chai, Yun Xiang, Ni Liao, Guofeng Gui, Ruo Yuan
DOI: 10.1039/C3AN01567J
A protein nanofiber hydrogel for sensitive immunoassays
Dae-Sung Lee, Jin-Seung Park, Eun Jung Lee, Hyun Jin Kim, Jeewon Lee
DOI: 10.1039/C3AN00564J
Multi-functional fluorescent probe for Hg2+, Cu2+ and ClO− based on a pyrimidin-4-yl phenothiazine derivative
Jiena Weng, Qunbo Mei, Bin Zhang, Yuanzhi Jiang, Bihai Tong, Quli Fan, Qidan Ling
DOI: 10.1039/C3AN01214J
A GFP-tagged nucleoprotein-based aggregation assay for anti-influenza drug discovery and antibody development
DOI: 10.1039/C3AN01041D
Realizing nano electrospray ionization using disposable pipette tips under super atmospheric pressure
Md. Matiur Rahman, Kenzo Hiraoka, Lee Chuin Chen
DOI: 10.1039/C3AN01635H
Effect of substrate choice and tissue type on tissue preparation for spectral histopathology by Raman microspectroscopy
Leanne M. Fullwood, Dave Griffiths, Katherine Ashton, Timothy Dawson, Robert W. Lea, Charles Davis, Franck Bonnier, Hugh J. Byrne, Matthew J. Baker
DOI: 10.1039/C3AN01832F
Development of a high-throughput assay for measuring lipase activity using natural triacylglycerols coated on microtiter plates
Carole Serveau-Avesque, Robert Verger, Jorge A. Rodriguez, Abdelkarim Abousalham
DOI: 10.1039/C3AN36699E