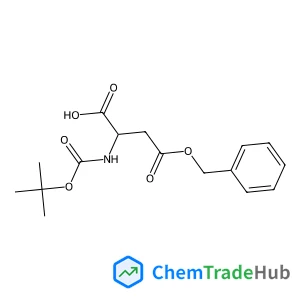
Boc-D-Asp(OBzl)-OH(CAS番号:51186-58-4)
ブ在玩家转储时丢失了部分字符,应为Boc-D-Asp(OBzl)-OH
基本情報
CAS番号
51186-58-4
分子式
C16H21NO6
分子量
323.34 g/mol
Quick Actions
基本的な物理特性
融点
101-105°C
沸点
508.1℃ at 760 mmHg
引火点
261.1℃
比旋光度
+18° to +21° (c=1 in DMF)
安全情報
安全情報を表示危険性の表示
H315; H319; H335
危険物区分
IRRITANT
同義語と参考文献
英語
- MFCD00038255
- HY-W004083
- AC-30045
- Boc-D-aspartic acid b-benzyl ester
- Boc-D-Asp(OBzl)-OH, >=98.0% (HPLC)
- D-Aspartic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 4-(phenylmethyl) ester
- (R)-4-(Benzyloxy)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid
- AKOS015995303
- N-alpha-t-Butyloxycarbonyl-D-aspartic acid beta-benzyl ester
- Boc-D-aspartic acid 4-benzyl ester
- J-300057
- N-Boc-D-aspartic acid O-benzyl ester
- beta-Benzyl N-(tert-butoxycarbonyl)-D-aspartate
- CS-W004083
- JPD6M53HLN
- (2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxo-4-phenylmethoxybutanoic acid
- Q-101774
- (R)-4-(Benzyloxy)-2-((tert-butoxycarbonyl)amino]-4-oxobutanoic acid
- Boc-D-Asp(OBzl)-OH
- (R)-2-tert-butoxycarbonylamino-succinic acid 4-benzyl ester
- 51186-58-4
- (R)-4-(Benzyloxy)-2-(tert-butoxycarbonylamino)-4-oxobutanoic acid
- O10178
- SOHLZANWVLCPHK-GFCCVEGCSA-N
- Boc-D-aspartic acid ?-benzyl ester
- 4-(Phenylmethyl) hydrogen N-[(1,1-dimethylethoxy)carbonyl]-D-aspartate
- AS-19226
- Boc-D-Asp(OBn)-OH
- Boc-D-Aspartic acid 4-benzyl ester
- Boc-D-Asp(OBzl)-OHBoc-D-aspartic acid β-benzyl ester
- BOC-D-ASPARTIC ACID BENZYL ESTER
- Boc-D-aspartic acid β-benzyl ester
- Boc-D-Asp-OBzl
- N-Alpha-t-Boc-D-aspartic acid beta-benzyl ester
- BOC-D-Aspartic acid beta-benzyl ester
MDL_Number
MFCD00038255
CAS番号
51186-58-4
Customs_Code
2924299090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. |
|||||
 中国 - Beijing Bailingwei Technology Co., Ltd. 中国 - Beijing Bailingwei Technology Co., Ltd. |
|||||
 中国 - Hubei Dahao Chemical Co., Ltd. 中国 - Hubei Dahao Chemical Co., Ltd. |
|||||
 中国 - Hubei Shixing Chemical Co., Ltd. 中国 - Hubei Shixing Chemical Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 スイス - Embion Technologies SA スイス - Embion Technologies SA |
|||||
 オーストリア - TIGER Coatings GmbH & Co. KG オーストリア - TIGER Coatings GmbH & Co. KG |
|||||
 中国 - Wuhan Eight Stars Biotechnology Co., Ltd. 中国 - Wuhan Eight Stars Biotechnology Co., Ltd. |
関連論文
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G