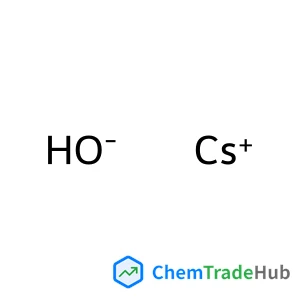
Caesium hydroxide | CAS No. 21351-79-1
Basic Information
CAS Number
21351-79-1
Molecular Formula
HCsO
Molecular Weight
149.91 g/mol
Quick Actions
Basic Physical Properties
Melting Point
272 °C(lit.)
Boiling Point
100 °C at 760 mmHg
Flash Point
°C
Solubility
Miscible with ethanol.
Vapor Pressure
No data available
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H302-H314
Hazard Class
8
Stability
Stable. Incompatible with acids. Absorbs carbon dioxide from the air.
Sensitiveness
Air Sensitive
Toxicity
LD50 i.p. in rats: 100 mg/kg (Cochran)
Synonyms & References
English
- Cesium hydroxide,hydrate(99.9%-cs)
- HUCVOHYBFXVBRW-UHFFFAOYSA-M
- Cesium hydroxide (Cs(OH))
- DTXSID7066699
- Caesium hydroxide [UN2682] [Corrosive]
- Cesium hydroxide monohydrate, 99.95% trace metals basis
- Cesium, di-mu-hydroxydi-
- J-014006
- NSC121987
- CESIUM HYDROXIDE(CS(OH)), HYDRATE (9CI)
- NSC 121987
- CESIUM HYDROXIDE [MI]
- 21351-79-1
- 12182-83-1
- HSDB 7906
- CsOH
- EINECS 244-344-1
- AKOS015904638
- CESIUM HYDROXIDE, HYDRATE (99.9%-CS)
- Cesium hydroxide monohydrate, >=99.5% trace metals basis
- Caesium hydroxide
- Q296363
- Cesium hydroxide
- UN2682
- NS00083783
- Cesium hydrate
- UNII-458ZFZ6235
- UN2681
- NSC-121987
- cesium;hydroxide
- Cesium hydroxide monohydrate, technical grade
- 458ZFZ6235
- MFCD00010964
- FT-0623567
- EC 244-344-1
- BP-30090
- CHEBI:33988
- caesium hydroxide
- Caesium hydrate
- Cesiumhydroxide (6CI,8CI)
- Cesium hydroxide solution
MDL_Number
MFCD00010964
CAS Number
21351-79-1
Customs_Code
2825909000
Merck_Index
13,2022
Recommended Suppliers
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shandong Gelon Lib Co., Ltd. China - Shandong Gelon Lib Co., Ltd. |
|||||
 China - Dezhou Jiatai Chemical Technology Co., Ltd. China - Dezhou Jiatai Chemical Technology Co., Ltd. |
|||||
 China - Jiangsu Shuangma Chemical Group China - Jiangsu Shuangma Chemical Group |
|||||
 Germany - Theion GmbH Germany - Theion GmbH |
|||||
 Spain - Materias Químicas, S.A. Spain - Materias Químicas, S.A. |
|||||
 China - Zhejiang Changxing Innovation Ultrafine Powder Co., Ltd. China - Zhejiang Changxing Innovation Ultrafine Powder Co., Ltd. |
|||||
 China - Chongqing Yuxi Medicine Technology Co., Ltd. China - Chongqing Yuxi Medicine Technology Co., Ltd. |
|||||
 China - Shanghai Hudong Boiler Factory China - Shanghai Hudong Boiler Factory |
Related Compounds
Related Articles
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A

![700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline 700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline](/structs/700/700874-71-1-fbbc.webp)
![315234-49-2 - 1-[(Tert-butoxy)carbonyl]-2-(prop-2-en-1-yl)pyrrolidine-2-carboxylic acid 315234-49-2 - 1-[(Tert-butoxy)carbonyl]-2-(prop-2-en-1-yl)pyrrolidine-2-carboxylic acid](/structs/315/315234-49-2-fe31.webp)