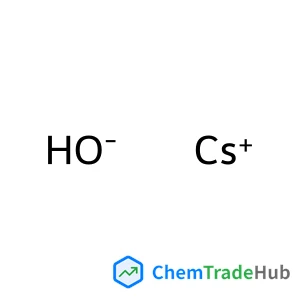
Caesium hydroxide(CAS号:21351-79-1)
氢氧化铯
基本信息
CAS号
21351-79-1
分子式
HCsO
分子量
149.91 g/mol
Quick Actions
基本物理性质
熔点
272 °C(lit.)
沸点
100 °C at 760 mmHg
闪点
°C
溶解度
Miscible with ethanol.
蒸气压
No data available
安全信息
查看安全信息危险说明
H302-H314
危险类别
8
稳定性
Stable. Incompatible with acids. Absorbs carbon dioxide from the air.
敏感性
Air Sensitive
毒性
LD50 i.p. in rats: 100 mg/kg (Cochran)
同义词与参考文献
英文
- Cesium hydroxide,hydrate(99.9%-cs)
- HUCVOHYBFXVBRW-UHFFFAOYSA-M
- Cesium hydroxide (Cs(OH))
- DTXSID7066699
- Caesium hydroxide [UN2682] [Corrosive]
- Cesium hydroxide monohydrate, 99.95% trace metals basis
- Cesium, di-mu-hydroxydi-
- J-014006
- NSC121987
- CESIUM HYDROXIDE(CS(OH)), HYDRATE (9CI)
- NSC 121987
- CESIUM HYDROXIDE [MI]
- 21351-79-1
- 12182-83-1
- HSDB 7906
- CsOH
- EINECS 244-344-1
- AKOS015904638
- CESIUM HYDROXIDE, HYDRATE (99.9%-CS)
- Cesium hydroxide monohydrate, >=99.5% trace metals basis
- Caesium hydroxide
- Q296363
- Cesium hydroxide
- UN2682
- NS00083783
- Cesium hydrate
- UNII-458ZFZ6235
- UN2681
- NSC-121987
- cesium;hydroxide
- Cesium hydroxide monohydrate, technical grade
- 458ZFZ6235
- MFCD00010964
- FT-0623567
- EC 244-344-1
- BP-30090
- CHEBI:33988
- caesium hydroxide
- Caesium hydrate
- Cesiumhydroxide (6CI,8CI)
- Cesium hydroxide solution
中文
- 氢氧化铯
- 氢氧化铯 溶液
MDL_Number
MFCD00010964
CAS号
21351-79-1
Customs_Code
2825909000
Merck_Index
13,2022
推荐供应商
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 台州新方向化工有限公司(原台州 中国 - 台州新方向化工有限公司(原台州 |
|||||
 中国 - 诸城泰盛化工股份有限公司 中国 - 诸城泰盛化工股份有限公司 |
|||||
 德国 - ARTES Valve & Service GmbH 德国 - ARTES Valve & Service GmbH |
|||||
 中国 - 上海太氟医药科技有限公司 中国 - 上海太氟医药科技有限公司 |
|||||
 德国 - 化学检验有限公司 德国 - 化学检验有限公司 |
|||||
 中国 - 武汉弘德悦欣医药科技有限公司 中国 - 武汉弘德悦欣医药科技有限公司 |
|||||
 中国 - 潍坊昌盛硝盐有限公司 中国 - 潍坊昌盛硝盐有限公司 |
|||||
 中国 - 宜兴市蓝星环保设备有限公司 中国 - 宜兴市蓝星环保设备有限公司 |
相关文献
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C

![28443-57-4 - 4-[4-[(5S)-5-(氨基甲基)-2-氧代-3-恶唑烷基]苯基]-3-吗啉酮 28443-57-4 - 4-[4-[(5S)-5-(氨基甲基)-2-氧代-3-恶唑烷基]苯基]-3-吗啉酮](/structs/284/28443-57-4-e4c7.webp)