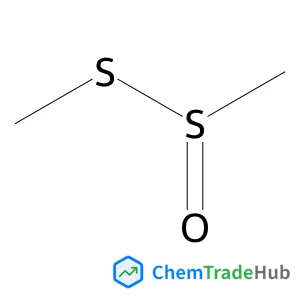
S-Methyl methanesulfinothioate | CAS No. 13882-12-7
Basic Information
CAS Number
13882-12-7
Molecular Formula
C2H6OS2
Molecular Weight
110.20 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
121.2°C
Refractive Index
1.5700 (estimate)
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- BRN 1738960
- S-methyl methane thiosulfinate
- S-methylmethane thiosulfinate
- NSC-23129
- SY359048
- CHEMBL403038
- EN300-213506
- Methanesulfinic acid, thio-, S-methyl ester (6CI,7CI,8CI)
- Dimethyl thiosulfinate
- 13882-12-7
- Methyl methanethiosulfinate
- CHEBI:184354
- Methyl methane thiosulphinate
- S-Methyl methanethiosulfinate
- RRGUMJYEQDVBFP-UHFFFAOYSA-N
- S-Methyl thiomethanesulfinate
- S-Methyl methanesulfinothioate
- (methanesulfinylsulfanyl)methane
- Dimethyldisulfide, S-oxide
- 4-04-00-00004 (Beilstein Handbook Reference)
- NSC23129
- BDBM50540965
- methylsulfinylsulfanyl-methane
- Methanesulfinothioic acid, S-methyl ester
- Me-SS(O)-Me
- methylsulinylsulanylmethane
- AKOS006278759
- NSC 23129
- DTXSID601310808
- C2H6OS2
- NS00093836
- methylsulfinylsulfanylmethane
- MFCD01754729
- SCHEMBL862565
- Methanesulfinic acid, thio-, S-methyl ester
- Methanesulfinothioicacid, S-methyl ester (9CI)
- methyl methanethiosulfinate
- dimethyl disulfide-S-monoxide
- Dimethyldisulfide,S-oxide
- dimethylthiosulfinate
- Methanesulfinothioic acid,S-methyl ester
- S-Methyl Methanethiosulfinate
- S-methyl methylthiosulfinate
- DIMETHYLTHIOSULPHINATE
- Methane methyl thiosulphinate
- Methyl(methylsulfinyl) sulfide
- Methanethiosulfinic acid S-methyl ester
MDL_Number
MFCD01754729
CAS Number
13882-12-7
Customs_Code
2930909090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. |
|||||
 China - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. China - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 China - Shanghai Kewei Chemical Technology Co., Ltd. China - Shanghai Kewei Chemical Technology Co., Ltd. |
|||||
 China - Guangdong Wanzhang Chemical Reagent Co., Ltd. China - Guangdong Wanzhang Chemical Reagent Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shijiazhuang Tianaoyue Fine Chemical Co., Ltd. China - Shijiazhuang Tianaoyue Fine Chemical Co., Ltd. |
|||||
 China - Shandong Luxi Animal Drug Co., Ltd China - Shandong Luxi Animal Drug Co., Ltd |
|||||
 China - San Yuan Jinrui Biological Engineering Co., Ltd. China - San Yuan Jinrui Biological Engineering Co., Ltd. |
Related Compounds
Recommended Journals
Related Articles
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Facile room-temperature growth of nanostructured CuBi2O4 for selective electrochemical reforming and photoelectrochemical hydrogen evolution reactions
Chia-Yu Lin, Shao-Yu Lin, Ming-Chun Tsai, Cheng-Hsien Wu
DOI: 10.1039/C9SE00558G
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementation
Akira Takai, Keiko Yoshizawa
DOI: 10.1039/C9CC08664A
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K

![221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate 221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate](/structs/221/221874-51-7-a692.webp)
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)