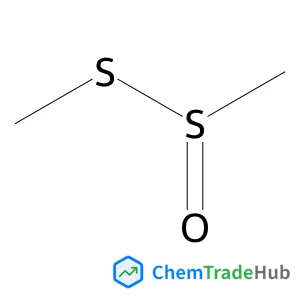
S-Methyl methanesulfinothioate(CAS番号:13882-12-7)
基本情報
CAS番号
13882-12-7
分子式
C2H6OS2
分子量
110.20 g/mol
Quick Actions
基本的な物理特性
沸点
121.2°C
屈折率
1.5700 (estimate)
安全情報
安全情報を表示同義語と参考文献
英語
- BRN 1738960
- S-methyl methane thiosulfinate
- S-methylmethane thiosulfinate
- NSC-23129
- SY359048
- CHEMBL403038
- EN300-213506
- Methanesulfinic acid, thio-, S-methyl ester (6CI,7CI,8CI)
- Dimethyl thiosulfinate
- 13882-12-7
- Methyl methanethiosulfinate
- CHEBI:184354
- Methyl methane thiosulphinate
- S-Methyl methanethiosulfinate
- RRGUMJYEQDVBFP-UHFFFAOYSA-N
- S-Methyl thiomethanesulfinate
- S-Methyl methanesulfinothioate
- (methanesulfinylsulfanyl)methane
- Dimethyldisulfide, S-oxide
- 4-04-00-00004 (Beilstein Handbook Reference)
- NSC23129
- BDBM50540965
- methylsulfinylsulfanyl-methane
- Methanesulfinothioic acid, S-methyl ester
- Me-SS(O)-Me
- methylsulinylsulanylmethane
- AKOS006278759
- NSC 23129
- DTXSID601310808
- C2H6OS2
- NS00093836
- methylsulfinylsulfanylmethane
- MFCD01754729
- SCHEMBL862565
- Methanesulfinic acid, thio-, S-methyl ester
- Methanesulfinothioicacid, S-methyl ester (9CI)
- methyl methanethiosulfinate
- dimethyl disulfide-S-monoxide
- Dimethyldisulfide,S-oxide
- dimethylthiosulfinate
- Methanesulfinothioic acid,S-methyl ester
- S-Methyl Methanethiosulfinate
- S-methyl methylthiosulfinate
- DIMETHYLTHIOSULPHINATE
- Methane methyl thiosulphinate
- Methyl(methylsulfinyl) sulfide
- Methanethiosulfinic acid S-methyl ester
MDL_Number
MFCD01754729
CAS番号
13882-12-7
Customs_Code
2930909090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hubei Yangxin Pharmaceutical Technology Co., Ltd. 中国 - Hubei Yangxin Pharmaceutical Technology Co., Ltd. |
|||||
 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 中国 - Shanghai Kewei Chemical Technology Co., Ltd. 中国 - Shanghai Kewei Chemical Technology Co., Ltd. |
|||||
 中国 - Guangdong Wanzhang Chemical Reagent Co., Ltd. 中国 - Guangdong Wanzhang Chemical Reagent Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Lai County Laiyu Chemical Industry Co., Ltd. 中国 - Lai County Laiyu Chemical Industry Co., Ltd. |
|||||
 ドイツ - DURAG GmbH ドイツ - DURAG GmbH |
|||||
 スイス - Rüegg F. スイス - Rüegg F. |
関連論文
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B

![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)

![37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione 37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione](/structs/378/37845-14-0-f8d0.webp)