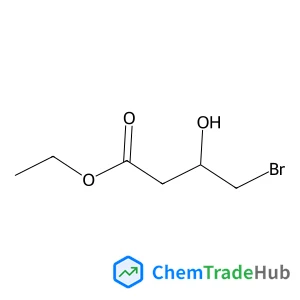
Ethyl (3S)-4-bromo-3-hydroxybutanoate(CAS号:95537-36-3)
(S)-4-溴-3-羟基丁酸乙酯
基本信息
CAS号
95537-36-3
分子式
C6H11BrO3
分子量
211.05 g/mol
Quick Actions
基本物理性质
沸点
94-96 °C2 mm Hg(lit.)
密度
1.468 g/mL at 25 °C(lit.)
闪点
>230 °F
折射率
n20/D 1.474(lit.)
安全信息
查看安全信息同义词与参考文献
英文
- AIZRKZQHJNWBEI-YFKPBYRVSA-N
- 95537-36-3
- (S)-Ethyl4-bromo-3-hydroxybutanoate
- ETHYL(S)-4-BROMO-3-HYDROXYBUTANOATE
- Butanoic acid,4-bromo-3-hydroxy-,ethyl ester,(3R)-
- AC-13919
- SCHEMBL1405832
- ETHYL (S)-4-BROMO-3-HYDROXYBUTANOATE
- (S)-4-BROMO-3-HYDROXYBUTYRIC ACID ETHYL ETHER
- CS-0370350
- ethyl (3S)-4-bromo-3-hydroxybutanoate
- Ethyl (S)-(-)-4-bromo-3-hydroxybutyrate
- ethyl (3s)-4-bromo-3-hydroxybutyrate
- Ethyl (S)-(-)-4-bromo-3-hydroxybutyrate, technical grade
- DTXSID90369976
- Ethyl-(S)-(-)-4-Bromo-3-hydroxybutanoate
- AKOS016842845
- ethyl (S)-4-bromo-3-hydroxybutyrate
- (S)-Ethyl 4-bromo-3-hydroxybutanoate
- Butanoic acid,4-bromo-3-hydroxy-,ethyl ester,(3S)-
- Butanoic acid,4-bromo-3-hydroxy-, ethyl ester, (3S)-
- Ethyl (S)-(?)-4-bromo-3-hydroxybutyrate
- (S)-4-bromo-3-hydroxybutyric acid ethyl ester
- (S)-bromo-3-hydroxybutyric acid ethy ester
- ethyl-(3S)-4-bromo-3-hydroxybutyrate
中文
- (S)-4-溴-3-羟基丁酸乙酯
- (S)-(-)-4-溴-3-羟丁酸乙酯
- (S)-4-溴-3-羟基丁酸乙醚
- 乙基-4-溴-3-羟基丁酯
- 乙基(3S)-4-溴-3-羟基丁酸酯
MDL_Number
MFCD00672875
CAS号
95537-36-3
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 苏州探科生物科技有限公司 中国 - 苏州探科生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海班科生物技术有限公司 中国 - 上海班科生物技术有限公司 |
|||||
 德国 - 波利西乌斯 AG 德国 - 波利西乌斯 AG |
|||||
 中国 - 长沙建运有色金属有限公司 中国 - 长沙建运有色金属有限公司 |
|||||
 中国 - 湖南纳昇电子科技有限公司 中国 - 湖南纳昇电子科技有限公司 |
相关文献
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Facile room-temperature growth of nanostructured CuBi2O4 for selective electrochemical reforming and photoelectrochemical hydrogen evolution reactions
Chia-Yu Lin, Shao-Yu Lin, Ming-Chun Tsai, Cheng-Hsien Wu
DOI: 10.1039/C9SE00558G
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B


![72050-71-6 - 2-基]-2,3-二羟箕-10,13-二甲箕-1,2,3,4,5,7,8,9,11,12,14,15,16,17-十四箐环戊烯并[a]菲-6 72050-71-6 - 2-基]-2,3-二羟箕-10,13-二甲箕-1,2,3,4,5,7,8,9,11,12,14,15,16,17-十四箐环戊烯并[a]菲-6](/structs/720/72050-71-6-6651.webp)