Ethyl (3S)-4-bromo-3-hydroxybutanoate(CAS番号:95537-36-3)
基本情報
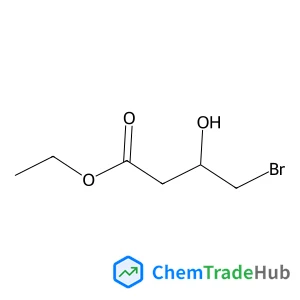
CAS番号
95537-36-3
分子式
C6H11BrO3
分子量
211.05 g/mol
Quick Actions
基本的な物理特性
沸点
94-96 °C2 mm Hg(lit.)
密度
1.468 g/mL at 25 °C(lit.)
引火点
>230 °F
屈折率
n20/D 1.474(lit.)
安全情報
安全情報を表示同義語と参考文献
英語
- AIZRKZQHJNWBEI-YFKPBYRVSA-N
- 95537-36-3
- (S)-Ethyl4-bromo-3-hydroxybutanoate
- ETHYL(S)-4-BROMO-3-HYDROXYBUTANOATE
- Butanoic acid,4-bromo-3-hydroxy-,ethyl ester,(3R)-
- AC-13919
- SCHEMBL1405832
- ETHYL (S)-4-BROMO-3-HYDROXYBUTANOATE
- (S)-4-BROMO-3-HYDROXYBUTYRIC ACID ETHYL ETHER
- CS-0370350
- ethyl (3S)-4-bromo-3-hydroxybutanoate
- Ethyl (S)-(-)-4-bromo-3-hydroxybutyrate
- ethyl (3s)-4-bromo-3-hydroxybutyrate
- Ethyl (S)-(-)-4-bromo-3-hydroxybutyrate, technical grade
- DTXSID90369976
- Ethyl-(S)-(-)-4-Bromo-3-hydroxybutanoate
- AKOS016842845
- ethyl (S)-4-bromo-3-hydroxybutyrate
- (S)-Ethyl 4-bromo-3-hydroxybutanoate
- Butanoic acid,4-bromo-3-hydroxy-,ethyl ester,(3S)-
- Butanoic acid,4-bromo-3-hydroxy-, ethyl ester, (3S)-
- Ethyl (S)-(?)-4-bromo-3-hydroxybutyrate
- (S)-4-bromo-3-hydroxybutyric acid ethyl ester
- (S)-bromo-3-hydroxybutyric acid ethy ester
- ethyl-(3S)-4-bromo-3-hydroxybutyrate
MDL_Number
MFCD00672875
CAS番号
95537-36-3
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Suzhou Tanke Biotechnology Co., Ltd. 中国 - Suzhou Tanke Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Bansu Biotechnology Co., Ltd. 中国 - Shanghai Bansu Biotechnology Co., Ltd. |
|||||
 ドイツ - MCI - Miritz Citrus Ingredients GmbH ドイツ - MCI - Miritz Citrus Ingredients GmbH |
|||||
 ドイツ - ABB AG ドイツ - ABB AG |
|||||
 ドイツ - STRIKO Verfahrenstechnik W.Strikfeldt & Koch GmbH ドイツ - STRIKO Verfahrenstechnik W.Strikfeldt & Koch GmbH |
おすすめジャーナル

Chemistry of Heterocyclic Compounds

Journal of the Chinese Chemical Society

Journal of the American Chemical Society

Australian Journal of Chemistry

Chemical & Pharmaceutical Bulletin

Doklady Chemistry

Canadian Metallurgical Quarterly

Chemistry of Natural Compounds

Bulletin of the Chemical Society of Japan

Advances in Colloid and Interface Science
関連論文
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems
Hasan Baig, Hiroyuki Kanda, Abdullah M. Asiri, Mohammad Khaja Nazeeruddin, Tapas Mallick
DOI: 10.1039/C9SE00550A
Synthesis and optical and electronic properties of one-dimensional sulfoxonium-based hybrid metal halide (CH3)3SOPbI3
Shiqiang Bai, Xizu Wang, Si Yin Tee, Siew Lay Lim, Lin Ke, Surani B. Dolmanan, Coryl Jing Jun Lee, Poh Chong Lim, Xiang Yao, Jishan Wu
DOI: 10.1039/D1CC01386F