EGFR/ErbB-2/ErbB-4 Inhibitor II(CAS号:944341-54-2)
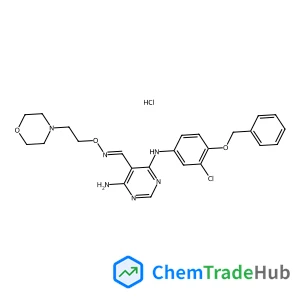
Jnj 28871063 hydrochloride
基本信息
CAS号
944341-54-2
分子式
C24H28Cl2N6O3
分子量
482.97 g/mol
Quick Actions
基本物理性质
安全信息
查看安全信息同义词与参考文献
英文
- 4-N-(3-chloro-4-phenylmethoxyphenyl)-5-(2-morpholin-4-ylethoxyiminomethyl)pyrimidine-4,6-diamine,hydrochloride
- 5E-4-Amino-6-(4-benzyloxy-3-chlorophenylamino)pyrimidine-5-carboxaldehydeN-(2-morpholin-4-ylethyl)oximehydrochloride
- JNJ 28871063 HYDROCHLORIDE
- EGFR/ErbB-2/ErbB-4 Inhibitor II
- 4-N-(3-chloro-4-phenylmethoxyphenyl)-5-[(E)-2-morpholin-4-ylethoxyiminomethyl]pyrimidine-4,6-diamine;hydrochloride
- 4-N-(3-chloro-4-phenylmethoxyphenyl)-5-(2-morpholin-4-ylethoxyiminomethyl)pyrimidine-4,6-diamine;hydrochloride
- N~4~-[4-(Benzyloxy)-3-chlorophenyl]-5-({[2-(morpholin-4-yl)ethoxy]imino}methyl)pyrimidine-4,6-diamine--hydrogen chloride (1/1)
- DTXSID70849569
- 944341-54-2
MDL_Number
MFCD12828762
CAS号
944341-54-2
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - Lyn Chemical 中国 - Lyn Chemical |
|||||
 德国 - 泰茨卡工业气体有限公司 德国 - 泰茨卡工业气体有限公司 |
|||||
 中国 - 衢州伟荣药化有限公司 中国 - 衢州伟荣药化有限公司 |
|||||
 中国 - 扬中沃顿机电有限公司 中国 - 扬中沃顿机电有限公司 |
|||||
 中国 - 上海芯上工业科技有限公司 中国 - 上海芯上工业科技有限公司 |
相关文献
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F

![224-53-3 - 二苯并[C,H]吖啶 224-53-3 - 二苯并[C,H]吖啶](/structs/224/224-53-3-97c9.webp)
![72050-71-6 - 2-基]-2,3-二羟箕-10,13-二甲箕-1,2,3,4,5,7,8,9,11,12,14,15,16,17-十四箐环戊烯并[a]菲-6 72050-71-6 - 2-基]-2,3-二羟箕-10,13-二甲箕-1,2,3,4,5,7,8,9,11,12,14,15,16,17-十四箐环戊烯并[a]菲-6](/structs/720/72050-71-6-6651.webp)