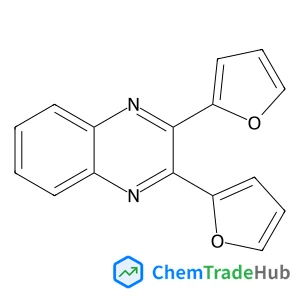
4-(2-Methyl-1H-imidazol-1-yl)aniline(CAS号:74852-81-6)
4-(2-甲基-1H-咪唑-1-基)苯胺
基本信息
CAS号
74852-81-6
分子式
C10H11N3
分子量
173.22 g/mol
Quick Actions
基本物理性质
熔点
111.5 °C
安全信息
查看安全信息危险说明
Harmful
危险类别
IRRITANT
同义词与参考文献
英文
- DTXSID40390134
- 4-(2-methyl-1H-imidazol-1-yl)aniline, AldrichCPR
- BDBM626110
- FT-0647264
- 4-(2-Methyl-1-imidazolyl)aniline
- 4-(2-methylimidazol-1-yl)aniline
- 4-(2-methyl-1H-imidazol-1-yl)benzenamine
- AKOS000143365
- SY079439
- 4-(2-Methyl-imidazol-1-yl)-phenylamine
- 4-(2-Methylimidazol-1-yl)phenylamine
- J-513325
- SCHEMBL265184
- IEZCMVRWKNEHJB-UHFFFAOYSA-N
- DS-10206
- CS-0152776
- 74852-81-6
- MFCD06797788
- 4-(2-methyl-1H-imidazol-1-yl)aniline
- 1-(4-aminophenyl)-2-methylimidazole
- Benzenamine, 4-(2-methyl-1H-imidazol-1-yl)-
- EN300-230665
- 4-(2-Methyl-1H-imidazol-1-yl)aniline
- 4-(2-Methyl-1H-imidazol-1-yl)phenylamine
- 4-(2-methylimidazolyl)phenylamine
- PubChem19934
- SBB014066
- STK350763
- 1-(4-aminopheny
中文
- 4-(2-甲基-1H-咪唑-1-基)苯胺
- 4-(2-甲基-1-咪唑)苯胺
MDL_Number
MFCD06797788
CAS号
74852-81-6
Customs_Code
2933290090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海修时生物医药有限公司 中国 - 上海修时生物医药有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - Manchester Organics Ltd 中国 - Manchester Organics Ltd |
|||||
 德国 - 康西克软件工程 德国 - 康西克软件工程 |
|||||
 中国 - Lyn Chemical 中国 - Lyn Chemical |
|||||
 德国 - Schwedes + Schulze Schüttgutttguttechnik GmbH 德国 - Schwedes + Schulze Schüttgutttguttechnik GmbH |
相关文献
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Hyosuke Mukohara, Hiroki Sato, Chihiro Tateishi, Hiromasa Sato
DOI: 10.1039/C9SE01068H
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B

![500789-05-9 - (Betar)-2-氯-Beta-[[(1,1-二甲基乙氧基)羰基]氨基]-苯丙酸 500789-05-9 - (Betar)-2-氯-Beta-[[(1,1-二甲基乙氧基)羰基]氨基]-苯丙酸](/structs/500/500789-05-9-80b4.webp)