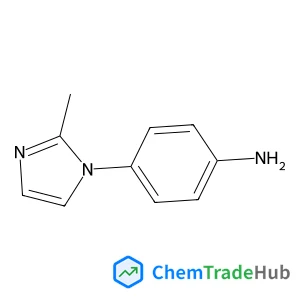
4-(2-Methyl-1H-imidazol-1-yl)aniline(CAS番号:74852-81-6)
基本情報
CAS番号
74852-81-6
分子式
C10H11N3
分子量
173.22 g/mol
Quick Actions
基本的な物理特性
融点
111.5 °C
安全情報
安全情報を表示危険性の表示
Harmful
危険物区分
IRRITANT
同義語と参考文献
英語
- DTXSID40390134
- 4-(2-methyl-1H-imidazol-1-yl)aniline, AldrichCPR
- BDBM626110
- FT-0647264
- 4-(2-Methyl-1-imidazolyl)aniline
- 4-(2-methylimidazol-1-yl)aniline
- 4-(2-methyl-1H-imidazol-1-yl)benzenamine
- AKOS000143365
- SY079439
- 4-(2-Methyl-imidazol-1-yl)-phenylamine
- 4-(2-Methylimidazol-1-yl)phenylamine
- J-513325
- SCHEMBL265184
- IEZCMVRWKNEHJB-UHFFFAOYSA-N
- DS-10206
- CS-0152776
- 74852-81-6
- MFCD06797788
- 4-(2-methyl-1H-imidazol-1-yl)aniline
- 1-(4-aminophenyl)-2-methylimidazole
- Benzenamine, 4-(2-methyl-1H-imidazol-1-yl)-
- EN300-230665
- 4-(2-Methyl-1H-imidazol-1-yl)aniline
- 4-(2-Methyl-1H-imidazol-1-yl)phenylamine
- 4-(2-methylimidazolyl)phenylamine
- PubChem19934
- SBB014066
- STK350763
- 1-(4-aminopheny
MDL_Number
MFCD06797788
CAS番号
74852-81-6
Customs_Code
2933290090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Xi Shi Biomedical Co., Ltd. 中国 - Shanghai Xi Shi Biomedical Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Manchester Organics Ltd 中国 - Manchester Organics Ltd |
|||||
 ドイツ - Battery Sphere GmbH ドイツ - Battery Sphere GmbH |
|||||
 スペイン - VAMEIN DE ESPAÑA, S.A. スペイン - VAMEIN DE ESPAÑA, S.A. |
|||||
 中国 - Shandong Yichun Chemical Co., Ltd. 中国 - Shandong Yichun Chemical Co., Ltd. |
おすすめジャーナル
関連論文
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
Synthesis and optical and electronic properties of one-dimensional sulfoxonium-based hybrid metal halide (CH3)3SOPbI3
Shiqiang Bai, Xizu Wang, Si Yin Tee, Siew Lay Lim, Lin Ke, Surani B. Dolmanan, Coryl Jing Jun Lee, Poh Chong Lim, Xiang Yao, Jishan Wu
DOI: 10.1039/D1CC01386F
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A



![56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol 56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol](/structs/568/56843-76-6-0035.webp)