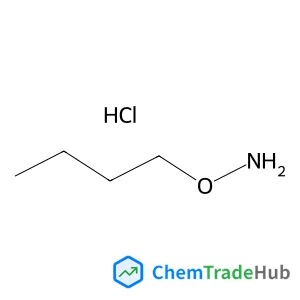
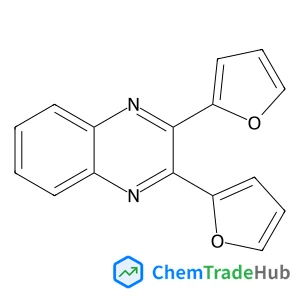
2,3-di(2-furyl)quinoxaline(CAS号:57490-73-0)
2,3-二(2-呋喃基)喹喔啉
基本信息
CAS号
57490-73-0
分子式
C16H10N2O2
分子量
262.26 g/mol
Quick Actions
基本物理性质
熔点
134-136 ºC
沸点
360.5±37.0 ºC (760 Torr),
密度
1.272±0.06 g/cm3 (20 ºC 760 Torr),
闪点
117.2±14.1 ºC,
安全信息
查看安全信息同义词与参考文献
英文
- AKOS000734524
- DTXSID90284711
- 2,3-bis(2-furyl)quinoxaline
- 2,3-di(furan-2-yl)quinoxaline
- cid_236275
- 2,3-di(2-furyl)quinoxaline
- 57490-73-0
- BDBM45345
- SR-01000394779-2
- 2,3-bis(furan-2-yl)quinoxaline
- MLS000097921
- Oprea1_689990
- AY-20590
- SR-01000394779
- SMR000060556
- 2,3-bis(2-furanyl)quinoxaline
- NSC-38592
- 2,3-di-2-furylquinoxaline
- Z55723200
- HMS547A15
- 2,3-Di-2-furanylquinoxaline
- CHEMBL305978
- CS-0694036
- NSC38592
- Maybridge1_001951
- CCG-49366
- HY-153349
- CBDivE_014398
- HMS2504J11
- Oprea1_075812
- SR-01000394779-1
- MLS-0057713.0001
- Quinoxaline,2,3-di-2-furanyl-
- 2,3-di(2-furanyl)quinoxaline
- 2,3'-di(2-furyl)-quinoxaline
- 2,3-di(furan-2-yl)quinoxalyne
- 2,3-di-furan-2-ylquinoxaline
- AC1L5W98
- AC1Q4WIR
- 2,3-Di-2-furylquinoxaline
- 2,3-Di(furan-2-yl)quinoxaline
中文
- 2,3-二(2-呋喃基)喹喔啉
CAS号
57490-73-0
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 合肥钼凯医药科技有限公司 中国 - 合肥钼凯医药科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 德国 - FWA Friedrich Werntges Apparatebau GmbH 德国 - FWA Friedrich Werntges Apparatebau GmbH |
|||||
 中国 - 诸城泰盛化工股份有限公司 中国 - 诸城泰盛化工股份有限公司 |
|||||
 中国 - 深圳优越昌浩科技有限公司 中国 - 深圳优越昌浩科技有限公司 |
相关文献
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
Interfacial engineering of a polymer–MOF composite by in situ vitrification
Rijia Lin, Jingwei Hou, Mengran Li, Zhanke Wang, Lei Ge, Shichun Li, Zhonghua Zhu, Thomas D. Bennett, Vicki Chen
DOI: 10.1039/D0CC00664E
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Hyosuke Mukohara, Hiroki Sato, Chihiro Tateishi, Hiromasa Sato
DOI: 10.1039/C9SE01068H
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA
Matthew R. Warren, Chenzhen Zhang, Armin Vedadghavami, Krister Bokvist, Pradeep K. Dhal
DOI: 10.1039/D0BM01497D
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F