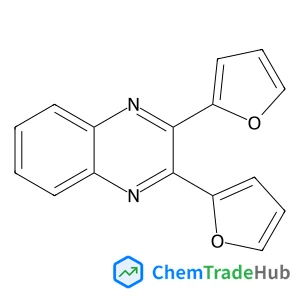
2,3-di(2-furyl)quinoxaline(CAS番号:57490-73-0)
基本情報
CAS番号
57490-73-0
分子式
C16H10N2O2
分子量
262.26 g/mol
Quick Actions
基本的な物理特性
融点
134-136 ºC
沸点
360.5±37.0 ºC (760 Torr),
密度
1.272±0.06 g/cm3 (20 ºC 760 Torr),
引火点
117.2±14.1 ºC,
安全情報
安全情報を表示同義語と参考文献
英語
- AKOS000734524
- DTXSID90284711
- 2,3-bis(2-furyl)quinoxaline
- 2,3-di(furan-2-yl)quinoxaline
- cid_236275
- 2,3-di(2-furyl)quinoxaline
- 57490-73-0
- BDBM45345
- SR-01000394779-2
- 2,3-bis(furan-2-yl)quinoxaline
- MLS000097921
- Oprea1_689990
- AY-20590
- SR-01000394779
- SMR000060556
- 2,3-bis(2-furanyl)quinoxaline
- NSC-38592
- 2,3-di-2-furylquinoxaline
- Z55723200
- HMS547A15
- 2,3-Di-2-furanylquinoxaline
- CHEMBL305978
- CS-0694036
- NSC38592
- Maybridge1_001951
- CCG-49366
- HY-153349
- CBDivE_014398
- HMS2504J11
- Oprea1_075812
- SR-01000394779-1
- MLS-0057713.0001
- Quinoxaline,2,3-di-2-furanyl-
- 2,3-di(2-furanyl)quinoxaline
- 2,3'-di(2-furyl)-quinoxaline
- 2,3-di(furan-2-yl)quinoxalyne
- 2,3-di-furan-2-ylquinoxaline
- AC1L5W98
- AC1Q4WIR
- 2,3-Di-2-furylquinoxaline
- 2,3-Di(furan-2-yl)quinoxaline
CAS番号
57490-73-0
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Huifei Mokai Medicinal Technology Co., Ltd. 中国 - Huifei Mokai Medicinal Technology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 アメリカ合衆国 - Zapata Computing, Inc. アメリカ合衆国 - Zapata Computing, Inc. |
|||||
 ドイツ - NETZSCH-Feinmahltechnik GmbH ドイツ - NETZSCH-Feinmahltechnik GmbH |
|||||
 オーストリア - Waagen Scheffknecht GmbH オーストリア - Waagen Scheffknecht GmbH |
関連論文
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Life cycle assessment of plasma-assisted ethylene production from rich-in-methane gas streams
Evangelos Delikonstantis, Elorri Igos, Michael Augustinus, Enrico Benetto
DOI: 10.1039/C9SE00736A

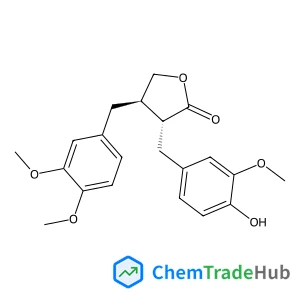
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)