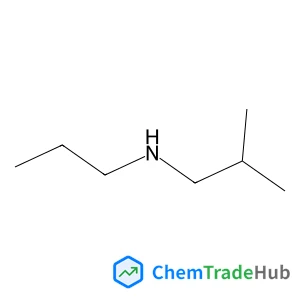
2-Methyl-n-propyl-1-propanamine(CAS号:39190-66-4)
丙基异丁胺
基本信息
CAS号
39190-66-4
分子式
C7H17N
分子量
115.22 g/mol
Quick Actions
基本物理性质
沸点
123-125 ºC (760 Torr)
闪点
16.3±9.3 ºC,
折射率
1.4065 (589.3 nm 20 ºC)
安全信息
查看安全信息同义词与参考文献
英文
- 2-methyl-N-propylpropan-1-amine
- 1-propanamine, 2-methyl-N-propyl-
- 2-Methyl-N-propyl-1-propanamine
- Isobutyl-propyl-amine
- Isobutyl-n-propylamine
- Isobutylpropylamine
- N-Isobutyl-N-propylamine
- (2-methylpropyl)(propyl)amine
- PROPYLISOBUTYLAMINE
- SQGSVBHTFQOZDL-UHFFFAOYSA-N
- Propylisobutyl amine
- propyl-isobutylamine
- n-propylisobutylamine
- propyl isobutyl amine
- n-Propyl-isobutylamin
- (2-Methyl-n-propyl)-1-propanamine
- 2
- 2-Methyl-N-propyl-1-pro
- AKOS000167254
- CS-11964
- SCHEMBL170842
- EN300-33331
- MFCD09929245
- 2-Methyl-N-propyl-1-propylamine, 9CI
- isobutylpropylamine
- Z90518190
- SY034221
- 39190-66-4
- N-isobutyl-N-propylamine
- DTXSID50879021
- DA-42549
- CHEBI:184419
- AC1640
- 2-Methyl-n-propyl-1-propanamine
- SCHEMBL9844731
中文
- 丙基异丁胺
MDL_Number
MFCD09929245
CAS号
39190-66-4
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海颖心实验室设备有限公司 中国 - 上海颖心实验室设备有限公司 |
|||||
 中国 - 杭州药睿易成生物医药有限公司 中国 - 杭州药睿易成生物医药有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 湖北汉威化工有限公司 中国 - 湖北汉威化工有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 瑞纳森(常州)国际贸易有限公司 中国 - 瑞纳森(常州)国际贸易有限公司 |
期刊推荐

Bulletin of the Chemical Society of Japan

Anti-Corrosion Methods and Materials

Ferroelectrics

Accounts of Chemical Research

Biopolymers

Journal of the American Chemical Society

Advances in Colloid and Interface Science

Canadian Metallurgical Quarterly

Australian Journal of Chemistry

Chemical & Pharmaceutical Bulletin
相关文献
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H