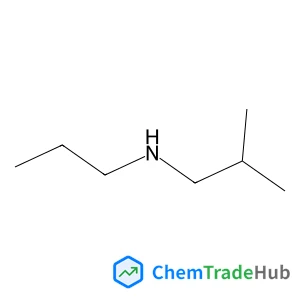
2-Methyl-n-propyl-1-propanamine(CAS番号:39190-66-4)
基本情報
CAS番号
39190-66-4
分子式
C7H17N
分子量
115.22 g/mol
Quick Actions
基本的な物理特性
沸点
123-125 ºC (760 Torr)
引火点
16.3±9.3 ºC,
屈折率
1.4065 (589.3 nm 20 ºC)
安全情報
安全情報を表示同義語と参考文献
英語
- 2-methyl-N-propylpropan-1-amine
- 1-propanamine, 2-methyl-N-propyl-
- 2-Methyl-N-propyl-1-propanamine
- Isobutyl-propyl-amine
- Isobutyl-n-propylamine
- Isobutylpropylamine
- N-Isobutyl-N-propylamine
- (2-methylpropyl)(propyl)amine
- PROPYLISOBUTYLAMINE
- SQGSVBHTFQOZDL-UHFFFAOYSA-N
- Propylisobutyl amine
- propyl-isobutylamine
- n-propylisobutylamine
- propyl isobutyl amine
- n-Propyl-isobutylamin
- (2-Methyl-n-propyl)-1-propanamine
- 2
- 2-Methyl-N-propyl-1-pro
- AKOS000167254
- CS-11964
- SCHEMBL170842
- EN300-33331
- MFCD09929245
- 2-Methyl-N-propyl-1-propylamine, 9CI
- isobutylpropylamine
- Z90518190
- SY034221
- 39190-66-4
- N-isobutyl-N-propylamine
- DTXSID50879021
- DA-42549
- CHEBI:184419
- AC1640
- 2-Methyl-n-propyl-1-propanamine
- SCHEMBL9844731
MDL_Number
MFCD09929245
CAS番号
39190-66-4
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. |
|||||
 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Hanwei Chemical Co., Ltd. 中国 - Hubei Hanwei Chemical Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 イギリス - Redd&Whyte Limited イギリス - Redd&Whyte Limited |
関連論文
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applications
Namachivayam Karthik, Tian Tian, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Yong Rok Lee, Seongbeom Kim, Dangsheng Xiong
DOI: 10.1039/C9SE00676A
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Synthesis and optical and electronic properties of one-dimensional sulfoxonium-based hybrid metal halide (CH3)3SOPbI3
Shiqiang Bai, Xizu Wang, Si Yin Tee, Siew Lay Lim, Lin Ke, Surani B. Dolmanan, Coryl Jing Jun Lee, Poh Chong Lim, Xiang Yao, Jishan Wu
DOI: 10.1039/D1CC01386F
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A

![500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid 500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid](/structs/500/500789-05-9-80b4.webp)