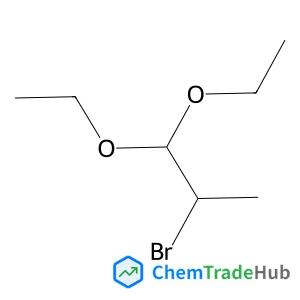
2-Bromo-1,1-diethoxypropane(CAS号:3400-55-3)
2-溴乙二醇二乙缩醛
基本信息
CAS号
3400-55-3
分子式
C7H15BrO2
分子量
211.10 g/mol
Quick Actions
基本物理性质
沸点
209.4°C at 760 mmHg
折射率
n20/D 1.439
安全信息
查看安全信息危险说明
H302-H315-H319-H335
同义词与参考文献
英文
- AHIUAFXWZKCJLR-UHFFFAOYSA-N
- 2-Bromopropionaldehyde diethyl acetal, 95%
- EN300-64677
- EINECS 222-270-0
- NS00049998
- bromopropanal diethyl acetal
- DTXSID00955581
- 2-Bromopriopionaldehydediethylacetal
- 2-bromopropionaldehyde diethyl acetal
- CS-0148273
- FT-0602642
- AMY33495
- SY025229
- J-508166
- 2-bromo-propionaldehyde-diethyl-acetal
- starbld0016321
- MFCD00152299
- 3400-55-3
- 2-Bromo-1,1-diethoxypropane
- DS-12668
- SCHEMBL5182048
- 3-HYDROXY-4-METHOXYPHENYLBORONICACID
- 2-BROMOPRIOPIONALDEHYDE DIETHYL ACETAL
- AKOS005137979
- Propane, 2-bromo-1,1-diethoxy-
- 2-Bromopriopionaldehyde diethyl acetyl
- Propane,2-bromo-1,1-diethoxy-
- 2-Bromopriopionaldehyde Diethyl Acetyl
- 2-Bromopropionaldehyde Diethyl Acetal
- 2-bromo-1,1-diethoxy-propane
- 2-bromopropanal ethyl acetal
- Propane,2-bromo-1,1-diethoxy
- C7H15BrO2
- STL557566
- BBL103756
- 2-BROMO-1,1-DIETHOXY PROPANE
- 2
中文
- 2-溴-1,1-二乙氧基丙烷
- 2-溴丙醛二乙缩醛
- 2-溴呋喃丙醛二甲缩醛
- 2-溴乙二醇二乙缩醛
MDL_Number
MFCD00152299
CAS号
3400-55-3
Customs_Code
2911000000
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 杭州药睿易成生物医药有限公司 中国 - 杭州药睿易成生物医药有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 湖北实兴化工有限公司 中国 - 湖北实兴化工有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 温州中树机械有限公司 中国 - 温州中树机械有限公司 |
相关文献
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems
Hasan Baig, Hiroyuki Kanda, Abdullah M. Asiri, Mohammad Khaja Nazeeruddin, Tapas Mallick
DOI: 10.1039/C9SE00550A
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D