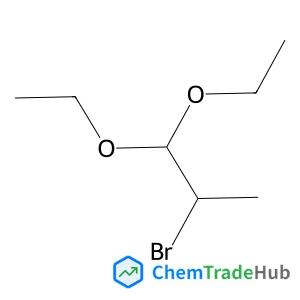
2-Bromo-1,1-diethoxypropane(CAS番号:3400-55-3)
基本情報
CAS番号
3400-55-3
分子式
C7H15BrO2
分子量
211.10 g/mol
Quick Actions
基本的な物理特性
沸点
209.4°C at 760 mmHg
屈折率
n20/D 1.439
安全情報
安全情報を表示危険性の表示
H302-H315-H319-H335
同義語と参考文献
英語
- AHIUAFXWZKCJLR-UHFFFAOYSA-N
- 2-Bromopropionaldehyde diethyl acetal, 95%
- EN300-64677
- EINECS 222-270-0
- NS00049998
- bromopropanal diethyl acetal
- DTXSID00955581
- 2-Bromopriopionaldehydediethylacetal
- 2-bromopropionaldehyde diethyl acetal
- CS-0148273
- FT-0602642
- AMY33495
- SY025229
- J-508166
- 2-bromo-propionaldehyde-diethyl-acetal
- starbld0016321
- MFCD00152299
- 3400-55-3
- 2-Bromo-1,1-diethoxypropane
- DS-12668
- SCHEMBL5182048
- 3-HYDROXY-4-METHOXYPHENYLBORONICACID
- 2-BROMOPRIOPIONALDEHYDE DIETHYL ACETAL
- AKOS005137979
- Propane, 2-bromo-1,1-diethoxy-
- 2-Bromopriopionaldehyde diethyl acetyl
- Propane,2-bromo-1,1-diethoxy-
- 2-Bromopriopionaldehyde Diethyl Acetyl
- 2-Bromopropionaldehyde Diethyl Acetal
- 2-bromo-1,1-diethoxy-propane
- 2-bromopropanal ethyl acetal
- Propane,2-bromo-1,1-diethoxy
- C7H15BrO2
- STL557566
- BBL103756
- 2-BROMO-1,1-DIETHOXY PROPANE
- 2
MDL_Number
MFCD00152299
CAS番号
3400-55-3
Customs_Code
2911000000
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Shixing Chemical Co., Ltd. 中国 - Hubei Shixing Chemical Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 ドイツ - Kern Technik GmbH & Co. KG ドイツ - Kern Technik GmbH & Co. KG |
関連論文
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J

![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)