Study of the effect of uranium recovery at Hap/Fe2O3 composite and Fe2O3 interfaces on the parameters of the electrical double layer
Literature Information
Adrianna Biedrzycka, Agnieszka Gładysz-Płaska, Ewa Skwarek
The electric double layer (edl) in the physicochemistry of colloids and electrochemistry is a term for a model of a structure appearing at the interface of two phases. The electrical double layer is of fundamental importance in the description of complex and common structures of matter. It is the reason for the stability of many colloidal systems. The aim of the study was to compare the experimental parameters characterizing the edl at Hap/Fe2O3 composite/electrolyte and Fe2O3/electrolyte interfaces in the presence of uranium ions and to compare the adsorption mechanisms. Hydroxyapatite itself is a well-known material used in many fields, including adsorption, medicine, and catalysis. However, additional modifications have provided it with additional beneficial features, such as low price, biodegradability, reactivity and stability, which can broaden the horizon of its applications. Iron oxide as a modifying agent gives the composite primarily magnetic properties that accelerate and facilitate its recovery from aqueous solutions. The paper presents the results of SEM, XRD, and XPS analyses and porosity measurement, as well as measurements of the surface charge density and zeta potential. Experiments confirmed the effectiveness of the synthesis process. In addition, the tests showed that the composite is characterized by a well-developed specific surface with a porous structure. The presence of uranium ions affects the experimentally determined parameters of the electrical double layer, such as: surface charge density and zeta potential. Uranium adsorption turned out to be a successful process, which confirms the possibility of using this material as an adsorbent. The structure of the adsorption layer formed on the synthetic composite and Fe2O3 surface changes significantly compared to the system with additionally present U(VI) ions. The adsorption of U(VI) ions causes a decrease in surface charge density and a decrease in zeta potential. Moreover, the adsorption of uranium resulted in the formation of a monolayer on the surface of Fe2O3 and Hap/Fe2O3 samples. However, the mechanism of the process is complex, meaning that adsorption can be physical as well as chemical. In addition, it should also be emphasized that uranium is a potentially toxic pollutant mainly associated with the nuclear industry. Therefore, there is a need to develop methods for its quick and safe removal from the environment.
Recommended Journals
Related Literature
IF 6.367
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteriesIF 6.367
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cellsIF 6.367
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineeringIF 6.843
Life cycle assessment of power-to-gas with biogas as the carbon sourceIF 6.367
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticlesIF 6.367
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layerIF 6.367
Outstanding Reviewers for ChemComm in 2020IF 6.222
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheetsIF 6.222
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in waterIF 6.222
Source Journal
Journal of Materials Chemistry A

Journal of Materials Chemistry A, B & C cover high quality studies across all fields of materials chemistry. The journals focus on those theoretical or experimental studies that report new understanding, applications, properties and synthesis of materials. The journals have a strong history of publishing quality reports of interest to interdisciplinary communities and providing an efficient and rigorous service through peer review and publication. The journals are led by an international team of Editors-in-Chief and Associate Editors who are all active researchers in their fields. Journal of Materials Chemistry A, B & C are separated by the intended application of the material studied. Broadly, applications in energy and sustainability are of interest to Journal of Materials Chemistry A, applications in biology and medicine are of interest to Journal of Materials Chemistry B, and applications in optical, magnetic and electronic devices are of interest to Journal of Materials Chemistry C. More than one Journal of Materials Chemistry journal may be suitable for certain fields and researchers are encouraged to submit their paper to the journal that they feel best fits for their particular article. Example topic areas within the scope of Journal of Materials Chemistry A are listed below. This list is neither exhaustive nor exclusive. Artificial photosynthesis Batteries Carbon dioxide conversion Catalysis Fuel cells Gas capture/separation/storage Green/sustainable materials Hydrogen generation Hydrogen storage Photocatalysis Photovoltaics Self-cleaning materials Self-healing materials Sensors Supercapacitors Thermoelectrics Water splitting Water treatment
Recommended Compounds
Recommended Suppliers
 Qingdao Xinxingtai Biotechnology Co., Ltd.
Qingdao Xinxingtai Biotechnology Co., Ltd. KIMESSA AG
KIMESSA AG Wuhan Eight Stars Biotechnology Co., Ltd.
Wuhan Eight Stars Biotechnology Co., Ltd. QUANCOM Informationssysteme GmbH
QUANCOM Informationssysteme GmbH Chromatographie-Zubehör Trott
Chromatographie-Zubehör Trott Wuzhou Oriental Technology Development Co., Ltd
Wuzhou Oriental Technology Development Co., Ltd Eurofins GfA Gesellschaft für Arbeitsplatz und Umweltanalytik mbH
Eurofins GfA Gesellschaft für Arbeitsplatz und Umweltanalytik mbH Jilin City Lin Xin Trade and Commerce Co., Ltd.
Jilin City Lin Xin Trade and Commerce Co., Ltd. Yangzhou TianTai Chemical Co., Ltd.
Yangzhou TianTai Chemical Co., Ltd. GB-Chemie GmbH
GB-Chemie GmbH










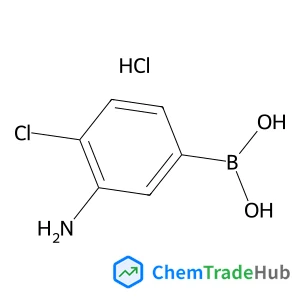
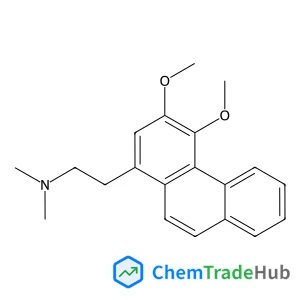
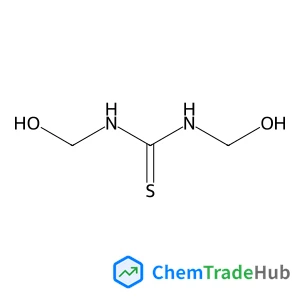
![697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate 697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate](/structs/697/697235-38-4-ee3a.webp)
![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)