Structural advances toward understanding the catalytic activity and conformational dynamics of modular nonribosomal peptide synthetases
Literature Information
Ketan D. Patel, Monica R. MacDonald, Syed Fardin Ahmed, Jitendra Singh, Andrew M. Gulick
Covering: up to fall 2022. Nonribosomal peptide synthetases (NRPSs) are a family of modular, multidomain enzymes that catalyze the biosynthesis of important peptide natural products, including antibiotics, siderophores, and molecules with other biological activity. The NRPS architecture involves an assembly line strategy that tethers amino acid building blocks and the growing peptides to integrated carrier protein domains that migrate between different catalytic domains for peptide bond formation and other chemical modifications. Examination of the structures of individual domains and larger multidomain proteins has identified conserved conformational states within a single module that are adopted by NRPS modules to carry out a coordinated biosynthetic strategy that is shared by diverse systems. In contrast, interactions between modules are much more dynamic and do not yet suggest conserved conformational states between modules. Here we describe the structures of NRPS protein domains and modules and discuss the implications for future natural product discovery.
Recommended Journals
Related Literature
IF 6.367
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layersIF 6.367
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloadditionIF 6.222
Strong circularly polarized luminescence of an octahedral chromium(iii) complexIF 6.222
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generationIF 6.367
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalystsIF 6.367
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexesIF 6.222
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapyIF 6.843
Photoactivatable fluorophores for durable labelling of individual cellsIF 6.222
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring systemIF 6.367
Source Journal
Natural Product Reports

Natural Product Reports (NPR) is a critical review journal that stimulates progress in all areas of natural products research, including isolation, structural and stereochemical determination, biosynthesis, biological activity and synthesis. The scope of the journal is very broad, and many reviews discuss the role of natural products in the wider bioinorganic, bioorganic and chemical biology communities. Areas covered include the following: Enzymology Nucleic acids Genetics Chemical ecology Carbohydrates Primary and secondary metabolism Analytical techniques NPR articles are designed to give an interesting insight into the topic, focusing on the key developments that have shaped a field. Authors are encouraged to include their own perspective on developments, trends and future directions. Articles providing a very comprehensive overview or exhaustive list of previous literature and lacking critical insight are generally not suitable for publication in NPR. Meta-analyses of previously published data using existing tools can be included, however NPR articles should not include any new methods or data.
Recommended Compounds
Recommended Suppliers
 Changzhou KaiKang Biological Science and Technology Co., Ltd.
Changzhou KaiKang Biological Science and Technology Co., Ltd. Comercio y Representaciones SA de CV (Coresa de CV)
Comercio y Representaciones SA de CV (Coresa de CV) PMA Prozess- und Maschinen-Automation GmbH
PMA Prozess- und Maschinen-Automation GmbH Liaoning Huaxing Corporation Limited
Liaoning Huaxing Corporation Limited Thermolab Scientific Equipments Pvt. Ltd.
Thermolab Scientific Equipments Pvt. Ltd. Hangzhou Rongda Pharmaceutical Chemical Co., Ltd.
Hangzhou Rongda Pharmaceutical Chemical Co., Ltd. QUANCOM Informationssysteme GmbH
QUANCOM Informationssysteme GmbH Nanolope
Nanolope Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd.
Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd. Wuhu Thompson Biotechnology Co., Ltd
Wuhu Thompson Biotechnology Co., Ltd










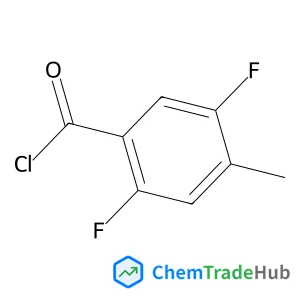

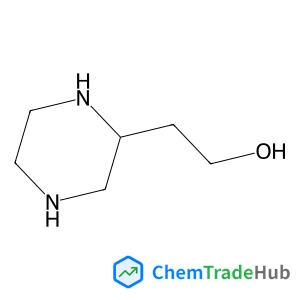
![697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate 697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate](/structs/697/697235-38-4-ee3a.webp)
