Chiral adaptive recognition with sequence specificity of aromatic dipeptides in aqueous solution by an achiral cage
Literature Information
Lin Cheng, Ping Tian, Honghong Duan, Qingfang Li, Xiaowen Song, Anyang Li, Liping Cao
Sequence-specific recognition of peptides and proteins by synthetic compounds or systems remains a huge challenge in biocompatible media. Here, we report the chiral adaptive recognition (CAR) with sequence specificity of aromatic dipeptides in a purely aqueous solution using an achiral tetraphenylethene-based octacationic cage (1) as both a molecular receptor and chiroptical sensor. 1 can selectively bind and dimerize aromatic dipeptides to form 1 : 2 host–guest complexes with high binding affinity (>1010 M−2), especially up to ∼1014 M−2 for TrpTrp. Given the dynamic rotational conformation of TPE units, achiral 1 can exhibit chiral adaptive responses with mirror-symmetrical circular dichroism (CD) and circularly polarized luminescence (CPL) spectra to enantiomeric dipeptides via supramolecular chirality transfer in the host–guest complexes. Furthermore, this CAR with sequence specificity of 1 can be applied for molecular recognition of TrpTrp- or PhePhe-containing tetrapeptides, polypeptides (e.g., amyloid β-peptide1–20 and somatostatin), and proteins (e.g., human insulin) with characteristic CD responses.
Related Literature
IF 6.222
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡IF 6.222
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalksIF 6.367
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reactionIF 6.367
A model-based comparison of Ru and Ni catalysts for the Sabatier reactionIF 6.367
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis processIF 6.367
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complexIF 6.222
Electrospun hydrogels for dynamic culture systems: advantages, progress, and opportunitiesIF 6.843
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexesIF 6.222
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a reviewIF 6.222
Source Journal
Chemical Science

Our journal has a wide-ranging scope which covers the full breadth of the chemical sciences. The research we publish contains the sorts of novel ideas, challenging questions and progressive thinking that bring undiscovered breakthroughs within reach. Your paper could focus on a single area, or cross many. It could be beyond the accepted bounds of the chemical sciences. It might address an immediate challenge, contribute to a future breakthrough or be wholly conceptual. We’re a team from every field of the chemical sciences, and know from experience that breakthroughs that drive the solutions to global challenges can come from anywhere, at any time. You could even start an entirely new area of research. Too bold? Too progressive? No such thing
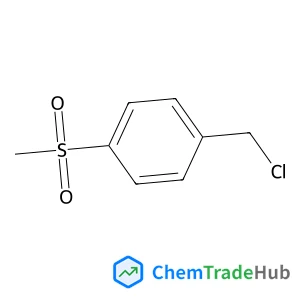
Recommended Compounds
Recommended Suppliers
 Hangzhou Hangyang Small Compressed Air Machine Co., Ltd.
Hangzhou Hangyang Small Compressed Air Machine Co., Ltd. Wenzhou Zhongshu Machinery Co., Ltd.
Wenzhou Zhongshu Machinery Co., Ltd. Heshi CHEM Co., Ltd (Hchte)
Heshi CHEM Co., Ltd (Hchte) VAMEIN DE ESPAÑA, S.A.
VAMEIN DE ESPAÑA, S.A. Hebei Anping Zhongtian Chemical Factory
Hebei Anping Zhongtian Chemical Factory International Lab Supliers de México, S.A. de C.V.
International Lab Supliers de México, S.A. de C.V. Shanghai Desheng Pharmaceutical Technology Co., Ltd.
Shanghai Desheng Pharmaceutical Technology Co., Ltd. StarXin Commerce and Trade Co., Ltd.
StarXin Commerce and Trade Co., Ltd. WEVO-CHEMIE GmbH
WEVO-CHEMIE GmbH Shandong Xishangxi New Materials Shareholding Co., Ltd. (API)
Shandong Xishangxi New Materials Shareholding Co., Ltd. (API)