Visible-light-induced organocatalytic enantioselective N–H insertion of α-diazoesters enabled by indirect free carbene capture
Literature Information
Wengang Guo, Ying Zhou, Hongling Xie, Xin Yue, Feng Jiang, Hai Huang, Zhengyu Han
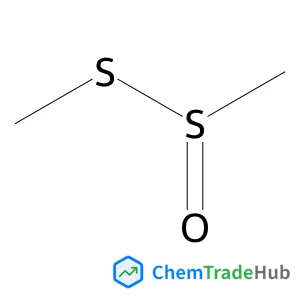
While asymmetric insertion of metal carbenes into H–X (X = C, N, O, etc.) bonds has been well-established, asymmetric control over free carbenes is challenging due to the presence of strong background reactions and lack of any anchor for a catalyst interaction. Here we have achieved the first photo-induced metal-free asymmetric H–X bond insertion of this type. With visible light used as a promoter and a chiral phosphoric acid used as a catalyst, α-diazoesters and aryl amines underwent smooth N–H bond insertion to form enantioenriched α-aminoesters with high efficiency and good enantioselectivity under mild conditions. Key to the success was the use of DMSO as an additive, which served to rapidly capture the highly reactive free carbene intermediate to form a domesticated sulfoxonium ylide.
Recommended Journals
Related Literature
IF 6.367
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteriesIF 6.367
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteriesIF 6.222
Contents listIF 6.843
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosisIF 6.222
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissueIF 6.222
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalystsIF 6.367
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cellsIF 6.367
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynesIF 6.222
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splittingIF 6.367
Source Journal
Chemical Science

Our journal has a wide-ranging scope which covers the full breadth of the chemical sciences. The research we publish contains the sorts of novel ideas, challenging questions and progressive thinking that bring undiscovered breakthroughs within reach. Your paper could focus on a single area, or cross many. It could be beyond the accepted bounds of the chemical sciences. It might address an immediate challenge, contribute to a future breakthrough or be wholly conceptual. We’re a team from every field of the chemical sciences, and know from experience that breakthroughs that drive the solutions to global challenges can come from anywhere, at any time. You could even start an entirely new area of research. Too bold? Too progressive? No such thing
Recommended Compounds
Recommended Suppliers
 FVG-Filter Vertriebs GmbH
FVG-Filter Vertriebs GmbH Jiangxi Wanxiangcu Biotechnology Co., Ltd.
Jiangxi Wanxiangcu Biotechnology Co., Ltd. INQUISA S.A.
INQUISA S.A. Ludwig Melosch Vertriebs-GmbH & Co.
Ludwig Melosch Vertriebs-GmbH & Co. AstroNova GmbH
AstroNova GmbH Lanxi Yongfeng Machinery Co., Ltd.
Lanxi Yongfeng Machinery Co., Ltd. Shijiazhuang Ultrafine New Materials Technology Co., Ltd.
Shijiazhuang Ultrafine New Materials Technology Co., Ltd. Lausitzer Analytik GmbH
Lausitzer Analytik GmbH Shandong Jingwei Pharmaceutical Co., Ltd.
Shandong Jingwei Pharmaceutical Co., Ltd. Hubei Xin Desheng Materials Technology Co., Ltd.
Hubei Xin Desheng Materials Technology Co., Ltd.










![224-53-3 - Dibenzo[c,h]acridine 224-53-3 - Dibenzo[c,h]acridine](/structs/224/224-53-3-97c9.webp)