The difference between photo-iniferter and conventional RAFT polymerization: high livingness enables the straightforward synthesis of multiblock copolymers
Literature Information
Jan A. M. Kurki
Photo-iniferter (PI)–RAFT polymerization, the direct activation of chain transfer agents via light, is a fascinating polymerization technique, as it overcomes some restriction of conventional RAFT polymerization. As such, we elucidated the role of reversible deactivation in this context using a monomer–CTA pair with low chain transfer capabilities. Tests with varying targeted degrees of polymerization (DP) or monomer concentrations revealed no significant improvement of polymerization control using the PI-process. Control can however be achieved via slow monomer addition, increasing the number of activation/deactivation events per monomer addition. More importantly, the livingness of the polymerization was found to be extraordinarily high, enabling the straightforward and rapid synthesis of multiblock copolymers with up to 20 blocks and a high number of repeating units per block (DP = 25–100) maintaining an overall excellent definition (Mn = 90 300 g mol−1, Đ = 1.29). This study highlights the enormous potential of PI–RAFT polymerization for the synthesis of polymeric materials.
Recommended Journals

Chemical Reviews

Anti-Corrosion Methods and Materials

Chemistry of Heterocyclic Compounds

Biopolymers

Australian Journal of Chemistry

Bulletin of the Chemical Society of Japan

Journal of the American Chemical Society

Chemical & Pharmaceutical Bulletin

Canadian Metallurgical Quarterly

Accounts of Chemical Research
Related Literature
IF 6.843
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complexIF 6.367
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in waterIF 6.222
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalystsIF 6.367
Sensitive and specific detection of tumour cells based on a multivalent DNA nanocreeper and a multiplexed fluorescence supersandwichIF 6.222
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complexIF 6.222
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheetsIF 6.222
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementationIF 6.222
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticlesIF 6.843
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissueIF 6.222
Source Journal
Polymer Chemistry

Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
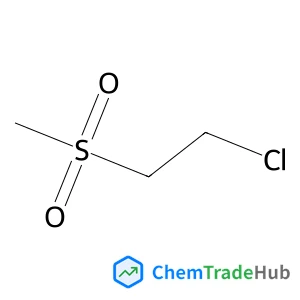
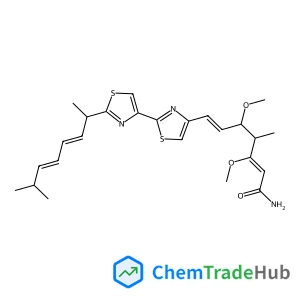
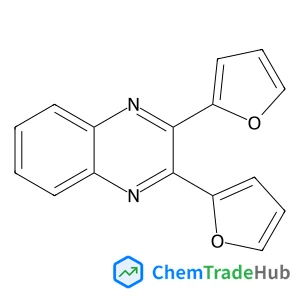
Recommended Compounds
Recommended Suppliers
 Hunan Fengen New Materials Technology Co., Ltd.
Hunan Fengen New Materials Technology Co., Ltd. Entegris, Inc.
Entegris, Inc. Shaanxi Tidu Medicine and Chemical Co., Ltd.
Shaanxi Tidu Medicine and Chemical Co., Ltd. Shandong Hehui International Trade Co., Ltd.
Shandong Hehui International Trade Co., Ltd. Luigs & Neumann Feinmechanik und Elektrotechnik GmbH
Luigs & Neumann Feinmechanik und Elektrotechnik GmbH Variati & Co. S.p.a.
Variati & Co. S.p.a. E.Georg Lüdecke Armaturen GmbH
E.Georg Lüdecke Armaturen GmbH Bureau Veritas Consumer Products Services Germany GmbH
Bureau Veritas Consumer Products Services Germany GmbH Zhenjiang Ht Chemical Industry Co., Ltd.
Zhenjiang Ht Chemical Industry Co., Ltd. Jinan Xinliye Chemical Industry Co., Ltd.
Jinan Xinliye Chemical Industry Co., Ltd.
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)