Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Literature Information
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
An addition/cyclization reaction of 1,6-diynes was developed for the synthesis of highly substituted 1,2-dialkylidenecycloalkanes. In this work, 1,6-diynes reacted with (dimethylphenylsilyl)pinacol-borane in the presence of a palladium catalyst to afford 1,2-dialkylidenecycloalkanes bearing silyl and boryl groups with a (Z,Z)-configuration in good to excellent yields. Moreover, the corresponding products could be easily converted into other synthetically useful compounds. This protocol provides an efficient and practical method of heteroelement–element linkage addition to the unsaturated 1,6-diynes.
Related Literature
IF 6.222
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approachIF 6.222
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromonesIF 6.222
Facile room-temperature growth of nanostructured CuBi2O4 for selective electrochemical reforming and photoelectrochemical hydrogen evolution reactionsIF 6.367
An aminophosphonate ester ligand-containing platinum(ii) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivoIF 6.222
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in waterIF 6.222
A new neodymium–phosphine compound for supercapacitors with long-term cycling stabilityIF 6.222
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring systemIF 6.367
Biomimetic hydrogels designed for cartilage tissue engineeringIF 6.843
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anodeIF 6.367
Source Journal
Chemical Communications

ChemComm publishes urgent research which is of outstanding significance and interest to experts in the field, while also appealing to the journal’s broad chemistry readership. Our communication format is ideally suited to short, urgent studies that are of such importance that they require accelerated publication. Our scope covers all topics in chemistry, and research at the interface of chemistry and other disciplines (such as materials science, nanoscience, physics, engineering and biology) where there is a significant novelty in the chemistry aspects. Major topic areas covered include: Analytical Chemistry Catalysis Chemical Biology and medicinal chemistry Computational Chemistry and Machine Learning Energy and sustainable chemistry Environmental Chemistry Green Chemistry Inorganic Chemistry Materials Chemistry Nanoscience Organic Chemistry Physical Chemistry Polymer Chemistry Supramolecular Chemistry
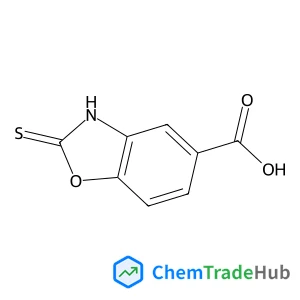
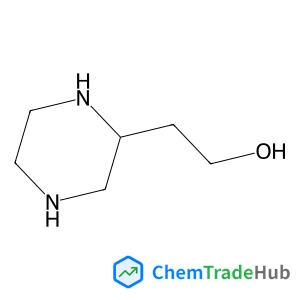
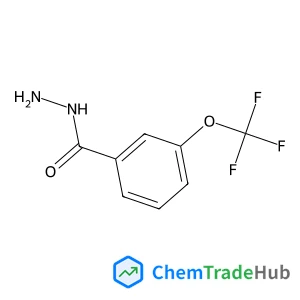
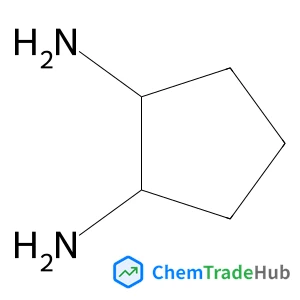
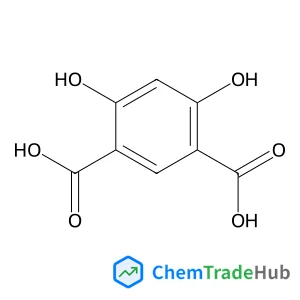
Recommended Compounds
Recommended Suppliers
 Medizintechnik PATZ GmbH
Medizintechnik PATZ GmbH Wuhan BoOutline Biotechnology Co., Ltd.
Wuhan BoOutline Biotechnology Co., Ltd. Hefei Novartis Biotechnology Co., Ltd.
Hefei Novartis Biotechnology Co., Ltd. Taizhou Jianjiang Tianyi Chemical Factory
Taizhou Jianjiang Tianyi Chemical Factory Nanjing Tri-Body Chemical Technology Co., Ltd.
Nanjing Tri-Body Chemical Technology Co., Ltd. Ningbo Zhenlei Chemical Co., Ltd.
Ningbo Zhenlei Chemical Co., Ltd. Sohena GmbH
Sohena GmbH Apexbio Technology LLC
Apexbio Technology LLC San Yuan Jinrui Biological Engineering Co., Ltd.
San Yuan Jinrui Biological Engineering Co., Ltd. Shijiazhuang Tianaoyue Fine Chemical Co., Ltd.
Shijiazhuang Tianaoyue Fine Chemical Co., Ltd.