Microfluidic serial dilution ladder
Literature Information
Siavash Ahrar, Michelle Hwang, Philip N. Duncan, Elliot E. Hui
Serial dilution is a fundamental procedure that is common to a large number of laboratory protocols. Automation of serial dilution is thus a valuable component for lab-on-a-chip systems. While a handful of different microfluidic strategies for serial dilution have been reported, approaches based on continuous flow mixing inherently consume larger amounts of sample volume and chip real estate. We employ valve-driven circulatory mixing to address these issues and also introduce a novel device structure to store each stage of the dilution process. The dilution strategy is based on sequentially mixing the rungs of a ladder structure. We demonstrate a 7-stage series of 1 : 1 dilutions with R2 equal to 0.995 in an active device area of 1 cm2.
Related Literature
IF 4.616
A novel and photostable pH probe for selectively staining nuclei in living cellsIF 4.616
Dual signal amplification of zinc oxide nanoparticles and quantum dots-functionalized zinc oxide nanoparticles for highly sensitive electrochemiluminescence immunosensingIF 4.616
On-chip screening for prostate cancer: an EIS microfluidic platform for contemporary detection of free and total PSAIF 4.616
A protein nanofiber hydrogel for sensitive immunoassaysIF 4.616
Peroxide induced ultra-weak chemiluminescence and its application in analytical chemistryIF 4.616
A low-cost thin layer coulometric microfluidic device based on an ion-selective membrane for calcium determinationIF 4.616
Microfluidic serial dilution ladderIF 4.616
Selective detection of complementarity-determining regions of monoclonal antibody by limiting protease access to the substrate: nano-surface and molecular-orientation limited proteolysisIF 4.616
Monitoring the mineralisation of bone nodules in vitro by space- and time-resolved Raman micro-spectroscopyIF 4.616
Source Journal
Analyst

Analyst publishes analytical and bioanalytical research that reports premier fundamental discoveries and inventions, and the applications of those discoveries, unconfined by traditional discipline barriers.
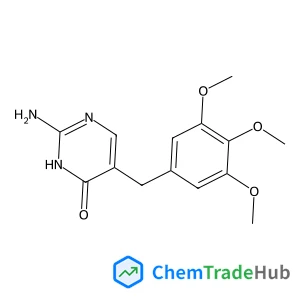
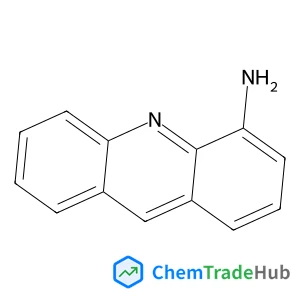
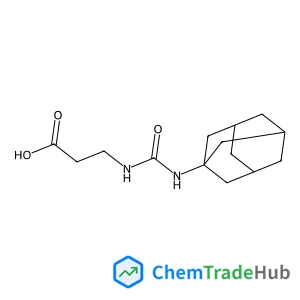
Recommended Compounds
Recommended Suppliers
 BHS-Sonthofen GmbH
BHS-Sonthofen GmbH Goudsche Machinefabriek B.V. (GMF-Gouda BV)
Goudsche Machinefabriek B.V. (GMF-Gouda BV) Beijing Baite Renkang Biopharmaceutical Technology Co., Ltd.
Beijing Baite Renkang Biopharmaceutical Technology Co., Ltd. Dycosa Resins S.L.
Dycosa Resins S.L. Lihang Rare Earth Industry Co., Ltd. Lianyungang City
Lihang Rare Earth Industry Co., Ltd. Lianyungang City Chengdu Boer Pharmaceutical Technology Co., Ltd.
Chengdu Boer Pharmaceutical Technology Co., Ltd. Kraton Polymers LLC
Kraton Polymers LLC Hangzhou Kenos Technology Biology Co., Ltd.
Hangzhou Kenos Technology Biology Co., Ltd. Daume Regelarmaturen GmbH
Daume Regelarmaturen GmbH Dongguan Tongzhou Chemical Co., Ltd.
Dongguan Tongzhou Chemical Co., Ltd.









![89483-07-8 - 3-Cyclopropyl-N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-alanine - N-cyclohexylcyclohexanamine (1:1) 89483-07-8 - 3-Cyclopropyl-N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-alanine - N-cyclohexylcyclohexanamine (1:1)](/structs/894/89483-07-8-8d6f.webp)
![884497-57-8 - 1-[2-(3,5-Dimethylphenoxy)ethyl]-1H-indole-3-carbaldehyde 884497-57-8 - 1-[2-(3,5-Dimethylphenoxy)ethyl]-1H-indole-3-carbaldehyde](/structs/884/884497-57-8-2fe5.webp)