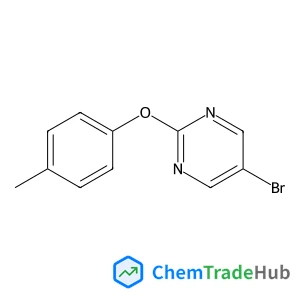
5-Bromo-2-(4-methylphenoxy)pyrimidine | CAS No. 887430-90-2
Basic Information
CAS Number
887430-90-2
Molecular Formula
C11H9BrN2O
Molecular Weight
265.11 g/mol
Quick Actions
Basic Physical Properties
Physical State
White to Yellow Solid
Melting Point
64-68 °C
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H302;H315;H320;H335
Synonyms & References
English
- MFCD03646338
- A923072
- 5-BROMO-2-P-TOLYLOXY-PYRIMIDINE
- FT-0704475
- 887430-90-2
- AKOS006283595
- CS-0172152
- SB58641
- AS-31363
- 5-bromo-2-(4-methylphenoxy)pyrimidine
- DTXSID50394542
- 5-bromo-2-(p-tolyloxy)pyrimidine
- Pyrimidine,5-bromo-2-(4-methylphenoxy)-
- 5-bromo-2-(4-methylphenoxy)Pyrimidine
- 5-BROMO-2-(P-TOLYLOXY)PYRIMIDINE
- A-1817
MDL_Number
MFCD03646338
CAS Number
887430-90-2
Customs_Code
2933599090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Yinxin Laboratory Equipment Co., Ltd. China - Shanghai Yinxin Laboratory Equipment Co., Ltd. |
|||||
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - L DV Water Power Star Biology and Technology Co., Ltd. China - L DV Water Power Star Biology and Technology Co., Ltd. |
|||||
 China - Weibo Changsheng Nitrate Co., Ltd. China - Weibo Changsheng Nitrate Co., Ltd. |
|||||
 Germany - KUKA Systems GmbH Germany - KUKA Systems GmbH |
|||||
 Germany - Dipl.-Ing. Wilhelm Schmidt GmbH Germany - Dipl.-Ing. Wilhelm Schmidt GmbH |
|||||
 China - Dezhou Jiatai Chemical Technology Co., Ltd. China - Dezhou Jiatai Chemical Technology Co., Ltd. |
|||||
 China - Nanjing Hoshin Pharmaceutical Technology Co., Ltd. China - Nanjing Hoshin Pharmaceutical Technology Co., Ltd. |
Related Compounds
Related Articles
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Electrospun hydrogels for dynamic culture systems: advantages, progress, and opportunities
M. Gregory Grewal
DOI: 10.1039/D0BM01588A
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems
Hasan Baig, Hiroyuki Kanda, Abdullah M. Asiri, Mohammad Khaja Nazeeruddin, Tapas Mallick
DOI: 10.1039/C9SE00550A
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B

![224-53-3 - Dibenzo[c,h]acridine 224-53-3 - Dibenzo[c,h]acridine](/structs/224/224-53-3-97c9.webp)
![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)