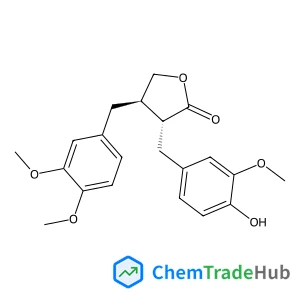
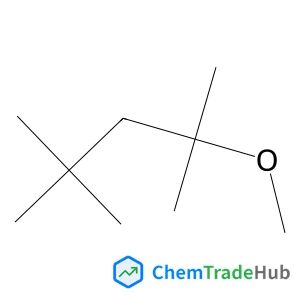
2-Methoxy-2,4,4-trimethylpentane | CAS No. 62108-41-2
Basic Information
CAS Number
62108-41-2
Molecular Formula
C9H20O
Molecular Weight
144.26 g/mol
Quick Actions
Basic Physical Properties
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- Pentane, 2-methoxy-2,4,4-trimethyl-
- Methyl 1,1,3,3-tetramethylbutyl ether
- [4-(2-bromoacetyl)phenyl]arsonic acid
- 2-Methoxy-2,4,4-trimethylpentane
- Methyl tert-octyl ether
- IKZVAPMTXDXWMX-UHFFFAOYSA-N
- 2-Methoxy-2,4,4-trimethylpentane #
- AKOS037645444
- 62108-41-2
- D93019
- AS-60793
- DTXSID001319426
- SCHEMBL43019
MDL_Number
MFCD18975889
CAS Number
62108-41-2
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Zhangjiajie Hengkang Biological Pharmaceutical Co., Ltd. China - Zhangjiajie Hengkang Biological Pharmaceutical Co., Ltd. |
|||||
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shanghai Yuyeh Biotechnology Co., Ltd. China - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 China - Shenzhen Chengfeng Intelligent Manufacturing Co., Ltd. China - Shenzhen Chengfeng Intelligent Manufacturing Co., Ltd. |
|||||
 Switzerland - Rüegg F. Switzerland - Rüegg F. |
|||||
 Germany - FVG-Filter Vertriebs GmbH Germany - FVG-Filter Vertriebs GmbH |
|||||
 China - Hefei Novartis Biotechnology Co., Ltd. China - Hefei Novartis Biotechnology Co., Ltd. |
Related Compounds
Related Articles
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H

![24449-39-6 - 2,2,2',2'-Tetramethyl-2H,2'H-5,5'-bibenzo[h]chromene-6,6'-diol 24449-39-6 - 2,2,2',2'-Tetramethyl-2H,2'H-5,5'-bibenzo[h]chromene-6,6'-diol](/structs/244/24449-39-6-3118.webp)