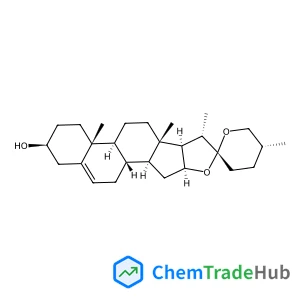
(3beta,25R)-Spirost-5-en-3-ol | CAS No. 512-04-9
Basic Information
CAS Number
512-04-9
Molecular Formula
C27H42O3
Molecular Weight
414.63 g/mol
Quick Actions
Basic Physical Properties
Physical State
Powder
Melting Point
205.0 to 208.0 deg-C
Boiling Point
527.1°C at 760 mmHg
Water Solubility
Soluble in chloroform (50 mg/ml), ethanol (83 mg/ml at 25°C), water (<1 mg/ml at 25°C), DMSO (<1 mg/ml at 25°C), and methanol.
Flash Point
272.6 °C
Solubility
chloroform: 20 mg/mL, clear, slightly yellow
Refractive Index
1.4700 (estimate)
Specific Rotation
D25 -129° (c = 1.4 in CHCl3)
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H302-H315-H319-H335
Storage Conditions
2-8℃
Synonyms & References
English
- Diosgenin
- HYDROXY-5-SPIROSTENE
- 3b-hydroxy-5-spirostene
- 3BETA-HYDROXY-5-SPIROSTENE
- 5,25R-SPIROSTEN-3BETA-OL
- 5,20-ALPHA, 22-ALPHA, 25D-SPIROSTEN-3-BETA-OL
- 5, 20, 22, 25D-SPIROSTEN-3-OL
- [25R]-5-SPIROSTEN-3BETA-OL
- Diosgenine
- DIOSGENIN (AS) PrintBack
- DIOSGENIN(SAPOGENIN)(P) PrintBack
- Spirost-5-en-3-ol,(3b,25R)-
- Wild Yam Extract Powder
- (25R)-5-Spirosten-3β-ol
- 3β-Hydroxy-5-spirostene
- DELTA 5,20A,22A,25D-SPIROSTEN-3BETA-OL
- Nitogenin
- (25R)-Spirost-5-en-3beta-ol
- [ "" ]
- 3β-hydroxy-5-spirostene; nitogenin
- K49P2K8WLX
- (25R)-spirost-5-en-3beta-diol
- (20R,25R)-Spirost-5-en-3beta-ol
- 22alpha-Spirost-5-en-3beta-ol
- SP 37
- (3beta,25R)-spirost-5-en-3-ol
- (25R)-5-Spirosten-3beta-ol
- nitrogen in
- Diosgenin,(S)
- (7S,8S,10S,13
Beilstein_Registry_Number
0094582
MDL_Number
MFCD00016887
CAS Number
512-04-9
Customs_Code
2932999099
Merck_Index
3295
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Sichuan Jingcuibao Biotechnology Co., Ltd. China - Sichuan Jingcuibao Biotechnology Co., Ltd. |
|||||
 China - Yiming Biotechnology (Shanghai) Co., Ltd China - Yiming Biotechnology (Shanghai) Co., Ltd |
|||||
 China - Wuhan Dingxin Tong Pharmaceutical Co., Ltd. China - Wuhan Dingxin Tong Pharmaceutical Co., Ltd. |
|||||
 China - Wuhan HuJiu Medicine Technology Co., Ltd. China - Wuhan HuJiu Medicine Technology Co., Ltd. |
|||||
 China - Shaanxi Diankai Biotechnology Co., Ltd. China - Shaanxi Diankai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Liken Chemical Technology Co., Ltd. China - Shanghai Liken Chemical Technology Co., Ltd. |
|||||
 Germany - AstroNova GmbH Germany - AstroNova GmbH |
|||||
 China - Liyi (Guangzhou) Material Technology Co., Ltd. China - Liyi (Guangzhou) Material Technology Co., Ltd. |
Related Compounds
Related Articles
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J

![37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione 37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione](/structs/378/37845-14-0-f8d0.webp)
![56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol 56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol](/structs/568/56843-76-6-0035.webp)









