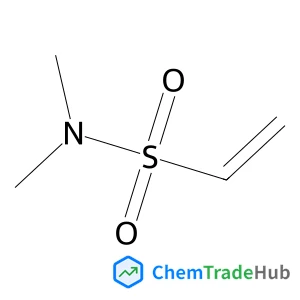
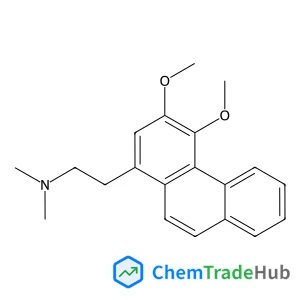
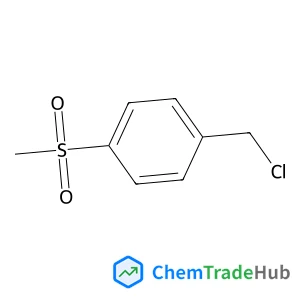
1-(Chloromethyl)-4-(methylsulfonyl)benzene | CAS No. 40517-43-9
Basic Information
CAS Number
40517-43-9
Molecular Formula
C8H9ClO2S
Molecular Weight
204.68 g/mol
Quick Actions
Basic Physical Properties
Melting Point
80 ºC
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Class
IRRITANT, LACHRYMATOR
Synonyms & References
English
- MFCD00079774
- Benzene, 1-(chloromethyl)-4-(methylsulfonyl)-
- p- methylsulfonylbenzyl chloride
- FT-0639648
- 1-(Chloromethyl)-4-(methylsulfonyl)benzene #
- 4-methylsulfonylbenzyl chloride
- 1-(chloromethyl)-4-methylsulfonylbenzene
- 1-(Chloromethyl)-4-(methanesulfonyl)benzene
- CK2015
- 40517-43-9
- p-Methylsulfonylbenzyl chloride
- 1-(chloromethyl)-4-methylsulfonyl-benzene
- DTXSID10960924
- 1-chloromethyl-4-(methanesulfonyl)-benzene
- 4-(methylsulfonyl)benzylchloride
- 4-(methylsulfonyl)benzyl chloride
- AS-45848
- p-methylsufonylbenzyl chloride
- 1-(chloromethyl)-4-methanesulfonylbenzene
- 5-(3-Hydroxy-phenyl)-isoxazole-3-carboxylicacid
- EN300-174490
- 1-chloromethyl-4-methanesulfonylbenzene
- alpha-Bromomethylparatolylsulfone
- SCHEMBL398570
- AKOS015852465
- 1-(Chloromethyl)-4-(methylsulfonyl)benzene
- A825150
- p-(Methylsulfonyl)benzyl chloride
- 4-methanesulfonylbenzyl chloride
- 4-Methylsulfonylbenzyl chloride
- 1-(chloromethyl)-4-(methylsulfonyl)Benzene
- 4-(METHYLSULFONYL)BENZYL CHLORIDE
- 1-Chloromethyl-4-methylsulfonylbenzene
- 1-Chloromethyl-4-methanesulfonylbenzene
- 4-Methanesulfonylbenzyl chloride
MDL_Number
MFCD00079774
CAS Number
40517-43-9
Customs_Code
2904909090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 China - Shanghai Yuyeh Biotechnology Co., Ltd. China - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 China - Liyang City Jiangdian Chemical Industry Co., Ltd. China - Liyang City Jiangdian Chemical Industry Co., Ltd. |
|||||
 Germany - E.Georg Lüdecke Armaturen GmbH Germany - E.Georg Lüdecke Armaturen GmbH |
|||||
 China - DYMATIC Chemicals Inc China - DYMATIC Chemicals Inc |
|||||
 China - Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd. China - Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd. |
Related Compounds
Related Articles
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementation
Akira Takai, Keiko Yoshizawa
DOI: 10.1039/C9CC08664A
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J