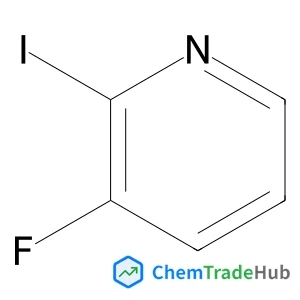
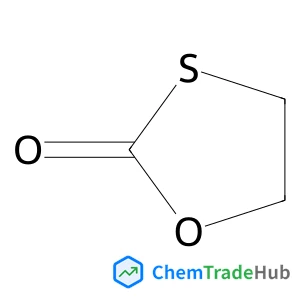
1,3-Oxathiolan-2-one | CAS No. 3326-89-4
Basic Information
CAS Number
3326-89-4
Molecular Formula
C3H4O2S
Molecular Weight
104.13 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
152.4°C at 760 mmHg
Density
1.374
Flash Point
68°C
Refractive Index
1.539
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- 1,3-oxathiolan-2-one
- 1,3-oxathiacyclopentan-2-one
- 1,3-oxathiolanone
- 2-Oxathiolone
- Ethanol,cyclic carbonate
- Ethylene monothiocarbonate
- monothiocarbonate d'ethylene
- oxathiolanne-1,3 one-2
- DTXSID4062981
- NSC 25761
- 3326-89-4
- Ethanol, cyclic carbonate
- SCHEMBL2014763
- G23825
- MFCD06797711
- 1,3-Oxathiolan-2-one
- NSC56813
- NSC-25761
- NSC-56813
- DAJDXKBYZZGECL-UHFFFAOYSA-N
- EN300-190268
- [1,3]-OXATHIOLAN-2-ONE
- NSC25761
- Ethanol, 2-mercapto-, cyclic carbonate
- NSC 56813
- AKOS006285024
- UNII-DD7J9EO5EA
- AI3-25353
- DD7J9EO5EA
MDL_Number
MFCD06797711
CAS Number
3326-89-4
Customs_Code
2934999090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hubei Hongxin Chemical Reagents Co., Ltd. China - Hubei Hongxin Chemical Reagents Co., Ltd. |
|||||
 China - Shanghai Kewei Chemical Technology Co., Ltd. China - Shanghai Kewei Chemical Technology Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 Germany - Paul Leibinger GmbH & Co. KG Germany - Paul Leibinger GmbH & Co. KG |
|||||
 Austria - M-Tech Fröschl, Maschinen- und Anlagenbau GmbH Austria - M-Tech Fröschl, Maschinen- und Anlagenbau GmbH |
|||||
 China - Tianjin Lichang Technology Development Co., Ltd. China - Tianjin Lichang Technology Development Co., Ltd. |
|||||
 China - Shanxi Chenshi ZhiGuang Chemical Technology Co., Ltd. China - Shanxi Chenshi ZhiGuang Chemical Technology Co., Ltd. |
|||||
 China - Quzhou Bangcheng Chemical Co., Ltd. China - Quzhou Bangcheng Chemical Co., Ltd. |
Related Compounds
Related Articles
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Hyosuke Mukohara, Hiroki Sato, Chihiro Tateishi, Hiromasa Sato
DOI: 10.1039/C9SE01068H
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D

![500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid 500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid](/structs/500/500789-05-9-80b4.webp)