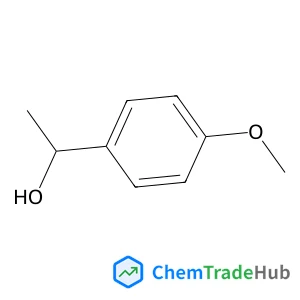
1-(4-Methoxyphenyl)ethanol | CAS No. 3319-15-1
Basic Information
CAS Number
3319-15-1
Molecular Formula
C9H12O2
Molecular Weight
152.19 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
95 °C/1 mmHg(lit.)
Water Solubility
Not miscible in water.
Refractive Index
n20/D 1.533(lit.)
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- SY114791
- D70953
- bmse010136
- FT-0622181
- (+/-)-1-(4-methoxyphenyl)ethanol
- (S)-alpha-Methyl-4-methoxybenzyl Alcohol
- .ALPHA.-METHYL-4-METHOXYBENZYL ALCOHOL
- alpha-methyl-4-methoxybenzyl alcohol
- A821654
- 4-methoxyphenyl ethanol
- 4-Methoxy-.alpha.-methylbenzyl alcohol
- Benzenemethanol, 4-methoxy-alpha-methyl-
- 4-Methoxy-alpha-methylbenzyl alcohol
- Q27159251
- NSC406712
- 1-(4-methoxyphenyl)-ethanol
- DS-11602
- AKOS016050377
- 1-(P-ANISYL)ETHANOL
- SY194794
- AI3-05520
- NS00048939
- 4-Methoxyphenyl methyl carbinol
- 3319-15-1
- Benzenemethanol, 4-methoxy-.alpha.-methyl-
- 1-(4-Methoxyphenyl)ethanol, (+/-)-
- 4-Methoxy- alpha -methylbenzyl alcohol
- FT-0688889
- Z335245134
- SY005068
- DTXSID401311686
- 018M85FD11
- SCHEMBL931283
- 1-(4-methoxyphenyl) ethanol
- (R)-alpha-Methyl-4-methoxybenzyl alcohol
- CHEBI:86566
- 4-Methoxy-alpha-methylbenzyl alcohol, 99%
- EN300-50553
- 1-(4-Methoxyphenyl)ethanol #
- AKOS000249013
- NSC 406712
- W-106785
- MFCD09863667
- EINECS 222-019-5
- UNII-018M85FD11
- 1-(4-methoxy-phenyl)ethanol
- 1-(4-methoxyphenyl)ethan-1-ol
- 1-(p-Methoxyphenyl)ethanol
- CS-0154099
- MFCD09863666
- 4-METHOXY-.ALPHA.-METHYLBENZENEMETHANOL
- MFCD00016857
- NSC-406712
- 1-(4-Methoxyphenyl)ethanol
- 4-methoxy-alpha-methylbenzyl alcohol
- 4-Methoxy-alpha-methylbenzyl alcohol~4-Methoxyphenyl methyl carbinol
- ALPHA-Methyl-p-anisyl alcohol
- 4-methoxyphenylethanol
- p-methoxy-1-phenyl ethanol
- p-Methoxyphenyl methyl carbinol
- alpha-Methyl-4-methoxybenzenemethanol
Beilstein_Registry_Number
2043521
MDL_Number
MFCD00016857
CAS Number
3319-15-1
Customs_Code
2909499000
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. |
|||||
 China - Guangdong Wanzhang Chemical Reagent Co., Ltd. China - Guangdong Wanzhang Chemical Reagent Co., Ltd. |
|||||
 China - Tianjin Bram Technology Co., Ltd. China - Tianjin Bram Technology Co., Ltd. |
|||||
 Germany - Interstuhl Büromöbel GmbH & Co. KG Germany - Interstuhl Büromöbel GmbH & Co. KG |
|||||
 Germany - DURAG GmbH Germany - DURAG GmbH |
|||||
 Germany - FVG-Filter Vertriebs GmbH Germany - FVG-Filter Vertriebs GmbH |
|||||
 China - Hainan PuLi Pharmaceutical Co., Ltd. China - Hainan PuLi Pharmaceutical Co., Ltd. |
|||||
 China - Shanghai Liken Chemical Technology Co., Ltd. China - Shanghai Liken Chemical Technology Co., Ltd. |
Related Compounds
Related Articles
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Sensitive and specific detection of tumour cells based on a multivalent DNA nanocreeper and a multiplexed fluorescence supersandwich
Lei Luo, Wenshan Li, Dongliu Xiang, Yanyun Ma, Yuan Zhou, Yaqing Xu, Nandi Chen, Qing Wang, Jin Huang, Jianbo Liu, Xiaohai Yang, Kemin Wang
DOI: 10.1039/C9CC08618H
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G

![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)









