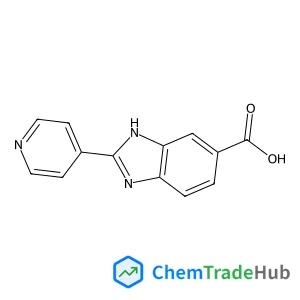
2-(4-Pyridinyl)-1H-benzimidazole-5-carboxylic acid | CAS No. 316833-32-6
Basic Information
CAS Number
316833-32-6
Molecular Formula
C13H9N3O2
Molecular Weight
239.23 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
575.2°C at 760 mmHg
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H315-H319-H335
Synonyms & References
English
- AKOS005257237
- A875831
- 2-(4-pyridinyl)-1H-benzimidazole-5-carboxylic acid
- AKOS002681109
- AMY23692
- DS-7485
- 2-(Pyridin-4-yl)-1H-benzo[d]imidazole-6-carboxylicacid
- CS-W021457
- F30027
- 2-pyridin-4-yl-1H-benzimidazole-5-carboxylic acid
- 2-pyridin-4-yl-3H-benzimidazole-5-carboxylic acid
- 2-(4-Pyridyl)benzimidazole-6-carboxylic acid
- 2-(4-Pyridinyl)-1H-benzimidazole-6-carboxylic acid
- 2-Pyridin-4-yl-3H-benzoimidazole-5-carboxylic acid, AldrichCPR
- 2-(Pyridin-4-yl)-1H-benzo[d]imidazole-6-carboxylic acid
- 316833-32-6
- DTXSID80378097
- 2-pyridin-4-yl-1H-benzimidazol-5-carboxylic acid
- MFCD06245477
- TYFICRRCLJYFJU-UHFFFAOYSA-N
- SCHEMBL4259320
- 2-(Pyridin-4-yl)-1H-benzimidazole-6-carboxylic acid
- YSWG042
- 2-pyridin-4-yl-3H-benzoimidazole-5-carboxylic Acid
- 1H-Benzimidazole-6-carboxylicacid, 2-(4-pyridinyl)-
- GL-0329
- 2-PYRIDIN-4-YL-3H-BENZOIMIDAZOLE-5-CARBOXYLIC ACID
- HPBCA
MDL_Number
MFCD06245477
CAS Number
316833-32-6
Customs_Code
2933990090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Beijing Bailingwei Technology Co., Ltd. China - Beijing Bailingwei Technology Co., Ltd. |
|||||
 China - Huifei Mokai Medicinal Technology Co., Ltd. China - Huifei Mokai Medicinal Technology Co., Ltd. |
|||||
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Tengzhun BioScience Co., Ltd. China - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shanghai Jixiang Biotechnology Co., Ltd. China - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 China - Shanghai Aladdin Bio-Technologies Co., Ltd. China - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 Germany - mentec GmbH Germany - mentec GmbH |
Related Compounds
Related Articles
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applications
Namachivayam Karthik, Tian Tian, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Yong Rok Lee, Seongbeom Kim, Dangsheng Xiong
DOI: 10.1039/C9SE00676A
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
An aminophosphonate ester ligand-containing platinum(ii) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivo
Ke-Bin Huang, Feng-Yang Wang, Hai-Wen Feng, Hejiang Luo, Yan Long, Albert S. C. Chan, Rong Liu, Huahong Zou, Zhen-Feng Chen, Yan-Cheng Liu, You-Nian Liu, Hong Liang
DOI: 10.1039/C9CC06563F

![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)