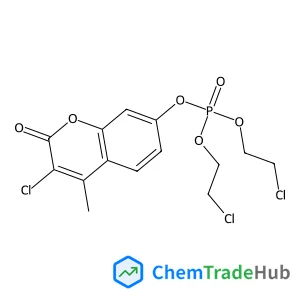
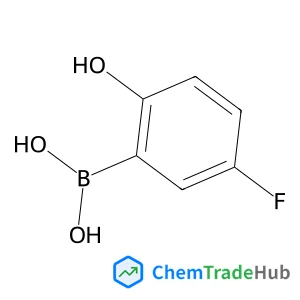
(5-Fluoro-2-hydroxyphenyl)boronic acid | CAS No. 259209-20-6
Basic Information
CAS Number
259209-20-6
Molecular Formula
C6H6BFO3
Molecular Weight
155.92 g/mol
Quick Actions
Basic Physical Properties
Physical State
White to Yellow Solid
Melting Point
160-166 °C
Boiling Point
347.1°C at 760 mmHg
Flash Point
163.7±30.7 °C
Refractive Index
1.552
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H315,H319,H335
Synonyms & References
English
- J-517505
- 259209-20-6
- 5-Fluoro-2-hydroxyphenylboronic acid
- CS-W020608
- (5-fluoro-2-hydroxyphenyl)boronic acid
- 5-fluoro-2-hydroxybenzeneboronic acid
- 5-fluoro-2-hydroxyphenyl-boronic acid
- Boronic acid, (5-fluoro-2-hydroxyphenyl)-
- EN300-6769050
- Boronic acid, B-(5-fluoro-2-hydroxyphenyl)-
- MFCD03095357
- TWDQSJDFXUMAOI-UHFFFAOYSA-N
- 5-Fluoro-2-hydroxyphenylboronic acid, AldrichCPR
- 5-fluoro-2-hydroxy phenyl boronic acid
- (5-fluoro-2-hydroxyphenyl)boronicacid
- FT-0647390
- DTXSID80396211
- SY047598
- Z1201622111
- (5-fluoro-2-hydroxy-phenyl)boronic Acid
- F0977
- PS-9529
- 5-fluoro-2-hydroxy-phenylboronic acid
- (5-fluoro-2-hydroxy)phenylboronic acid
- AM803598
- AKOS000285056
- SCHEMBL319589
- 2-hydroxy-5-fluorophenyl-boronic acid
- 2-hydroxy-5-fluorophenylboronic acid
- 5-fluoro-2-hydroxyphenylboronic acid (contains varying amounts of anhydride)
- AC-16596
- (5-Fluoro-2-hydroxyphenyl)boronic acid
- 5-Fluoro-2-hydroxyphenylboronic Acid (contains varying amounts of Anhydride)
- 4-FLUORO-2-HYDROXYPHENYLBORONIC ACID
- Boronic acid,B-(5-fluoro-2-hydroxyphenyl)-
- 2-Borono-4-fluorophenol
- 5-Fluoro-2-hydroxybenzeneboronic acid
- 5-Fluoro-2-hydroxybenzeneboronic Acid (contains varying amounts of Anhydride)
- PubChem19871
- 2-(Dihydroxyboryl)-4-fluorophenol
- 2-Hydroxy-5-fluorophenylboronic acid
MDL_Number
MFCD03095357
CAS Number
259209-20-6
Customs_Code
2931900090
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - East Asia Chemical Co., Ltd. Shandong China - East Asia Chemical Co., Ltd. Shandong |
|||||
 China - Hubei Fangde New Materials Co., Ltd. China - Hubei Fangde New Materials Co., Ltd. |
|||||
 China - Shanghai Jixiang Biotechnology Co., Ltd. China - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 China - Hubei Yunmei Technology Co., Ltd. China - Hubei Yunmei Technology Co., Ltd. |
|||||
 Germany - heatsystems GmbH & Co. KG Germany - heatsystems GmbH & Co. KG |
|||||
 China - Hangzhou Haidah Pharmaceutical Chemical Co., Ltd. China - Hangzhou Haidah Pharmaceutical Chemical Co., Ltd. |
|||||
 Germany - Edelstahlservice Sulz GmbH Germany - Edelstahlservice Sulz GmbH |
|||||
 China - Weibo Changsheng Nitrate Co., Ltd. China - Weibo Changsheng Nitrate Co., Ltd. |
Related Compounds
Related Articles
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A

![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)