N-[(Benzyloxy)carbonyl]-L-phenylalanyl-L-tryptophan | CAS No. 16856-28-3
Basic Information
![N-[(Benzyloxy)carbonyl]-L-phenylalanyl-L-tryptophan N-[(Benzyloxy)carbonyl]-L-phenylalanyl-L-tryptophan](/structs/168/16856-28-3-f319.webp)
CAS Number
16856-28-3
Molecular Formula
C28H27N3O5
Molecular Weight
485.54 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
819.3°Cat760mmHg
Density
1.316
Flash Point
449.3°C
Refractive Index
1.655
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- (S)-2-((S)-2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanamido)-3-(1H-indol-3-yl)propanoic acid
- 16856-28-3
- L-Tryptophan, N-[(phenylmethoxy)carbonyl]-L-phenylalanyl-
- Z-Phe-Trp-OH
- A810988
- (2S)-2-[(2S)-2-{[(BENZYLOXY)CARBONYL]AMINO}-3-PHENYLPROPANAMIDO]-3-(1H-INDOL-3-YL)PROPANOIC ACID
- (S)-2-((S)-2-(benzyloxycarbonylamino)-3-phenylpropanamido)-3-(1H-indol-3-yl)propanoic acid
- (S)-2-((S)-2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanamido)-3-(1H-indol-3-yl)propanoicacid
- benzyloxycarbonyl phenylalanyl-tryptophan
- ((Benzyloxy)carbonyl)-L-phenylalanyl-L-tryptophan
- CS-0447805
- 3-(1H-indol-3-yl)-2-[[3-phenyl-2-(phenylmethoxycarbonylamino)propanoyl]amino]propanoic acid
- L-Tryptophan,N-[N-[(phenylmethoxy)carbonyl]-L-phenylalanyl]- (9CI)
- Z-PHE-TRP-OH
- CBZ-L-PHE-TRP
- Cbz-Phe-trp-OH
- Z-Phe-Trp-OH99%
- Z-L-PHENYLALANYL-L-TRYPTOPHAN
MDL_Number
MFCD00191140
CAS Number
16856-28-3
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. China - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 China - Shanghai Jieshukai Biotechnology Co., Ltd. China - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shanghai Jixiang Biotechnology Co., Ltd. China - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 China - Shanghai Chupeptide Biotechnology Co., Ltd. China - Shanghai Chupeptide Biotechnology Co., Ltd. |
|||||
 China - Shanghai Aladdin Bio-Technologies Co., Ltd. China - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 China - Wuhan Eight Stars Biotechnology Co., Ltd. China - Wuhan Eight Stars Biotechnology Co., Ltd. |
|||||
 China - Tianjin Bochuang Chemical Co., Ltd. China - Tianjin Bochuang Chemical Co., Ltd. |
Related Compounds
Related Articles
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A

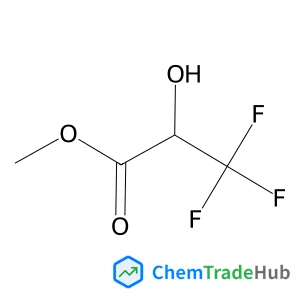
![221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate 221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate](/structs/221/221874-51-7-a692.webp)
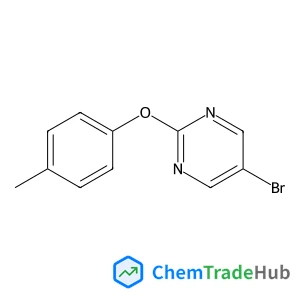
![697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate 697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate](/structs/697/697235-38-4-ee3a.webp)