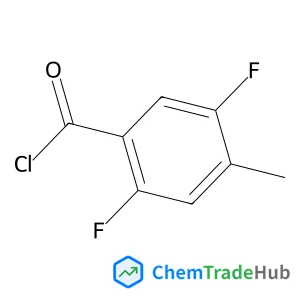
1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone | CAS No. 1337532-29-2
Basic Information
![1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone](/structs/133/1337532-29-2-edb6.webp)
CAS Number
1337532-29-2
Molecular Formula
C23H21FN6O
Molecular Weight
416.46 g/mol
Quick Actions
Basic Physical Properties
Boiling Point
744.6±60.0°C at 760 mmHg
Solubility
Insuluble (3.4E-3 g/L) (25 ºC),
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationHazard Statement
H302
Synonyms & References
English
- GSK2656157
- 1-(5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone
- 1-?[5-?(4-?amino-?7-?methyl-?7H-?pyrrolo[2,?3-?d]?pyrimidin-?5-?yl)?-?4-?fluoro-?2,?3-?dihyd
- GSK-2656157
- AK174904
- 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethan-1-one
- C23H21FN6O
- PRWSIEBRGXYXAJ-UHFFFAOYSA-N
- GTPL9544
- HMS3652P16
- AOB87106
- BCP09007
- s7033
- BDBM50442166
- 2448AH
- SB19409
- compound
- 1-[5-(4-Amino-7-methylpyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydroindol-1-yl]-2-(6-methylpyrid
- 1-[5-(4-Amino-7-methylpyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydroindol-1-yl]-2-(6-methylpyridin-2-yl)ethanone
- CHEMBL2441340
- 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone
- 1-[5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydro-1H-indol-1-yl]-2-(6-methyl-2-pyridinyl)-ethanone
- 1-[5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydro-1H-indol-1-yl]-2-(6-methyl-2-pyridinyl)ethanone
- PERK inhibitor GSK2656157
- HMS3868L13
- 1-(5-{4-AMINO-7-METHYLPYRROLO[2,3-D]PYRIMIDIN-5-YL}-4-FLUORO-2,3-DIHYDROINDOL-1-YL)-2-(6-METHYLPYRIDIN-2-YL)ETHANONE
- AKOS025404913
- NCGC00386314-01
- AC-32973
- SW222243-1
- NCGC00386314-03
- HY-13820
- 1-[5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydro-1H-indol-1-yl]-2-(6-methyl-2-pyridinyl)-ethanone; 5-[4-Fluoro-1-[(6-methyl-2-pyridinyl)acetyl]-2,3-dihydro-1H-indol-5-yl]-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- 5-{4-fluoro-1-[(6-methyl-2-pyridinyl)acetyl]-2,3-dihydro-1H-indol-5-yl}-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- CHEBI:139047
- 1337532-29-2
- J-006434
- AS-35165
- CCG-268839
- CS-3262
- 1-[5-(4-amino-7-methylpyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydroindol-1-yl]-2-(6-methylpyridin-2-yl)ethanone
- SCHEMBL870782
- compound 6 [PMID: 24900593]
- GSK 2656157
- DA-31727
- MFCD27997885
- AGN-PC-09SWN4
- EX-A439
- 5-{4-fluoro-1[(6-methyl-2-pyridinyl)acetyl]-2,3-dihydro-1H-indol-5-yl}-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- DTXSID101103941
- HMS3747M09
MDL_Number
MFCD27997885
CAS Number
1337532-29-2
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Beijing Solabe Technology Co., Ltd. China - Beijing Solabe Technology Co., Ltd. |
|||||
 China - Shanghai Mofu Medicine Technology Co., Ltd. China - Shanghai Mofu Medicine Technology Co., Ltd. |
|||||
 China - Shanghai Luofa Biochemical Technology Co., Ltd. China - Shanghai Luofa Biochemical Technology Co., Ltd. |
|||||
 China - Shanghai Hansi Chemical Co., Ltd. China - Shanghai Hansi Chemical Co., Ltd. |
|||||
 China - Shanghai Jixiang Biotechnology Co., Ltd. China - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 China - Jin Jinle (Hunan) Chemical Co., Ltd. China - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 China - Shanghai Yuansi Biotechnology Co., Ltd. China - Shanghai Yuansi Biotechnology Co., Ltd. |
|||||
 Germany - Dr. Reinschmidt Gefahrgutberatung Germany - Dr. Reinschmidt Gefahrgutberatung |
Related Compounds
Related Articles
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E

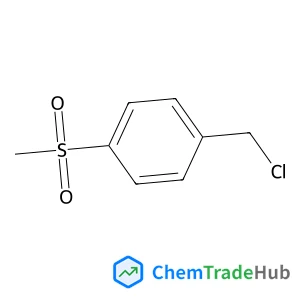
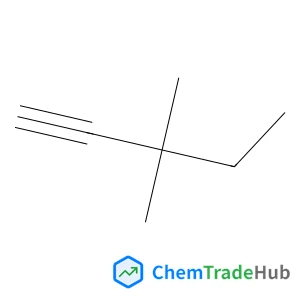
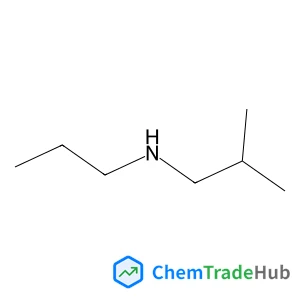
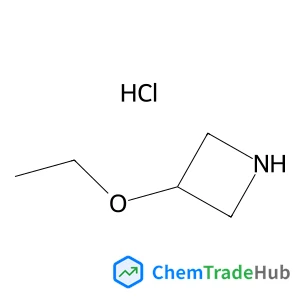
![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)
![224-53-3 - Dibenzo[c,h]acridine 224-53-3 - Dibenzo[c,h]acridine](/structs/224/224-53-3-97c9.webp)