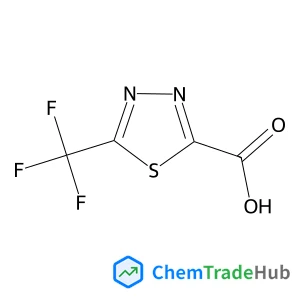
5-(trifluoromethyl)-1,3,4-thiadiazole-2-carboxylic acid | CAS No. 1260665-07-3
Basic Information
CAS Number
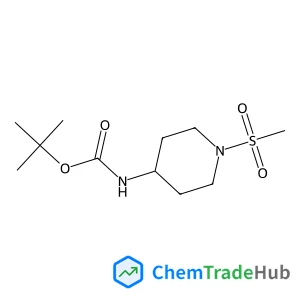
1260665-07-3
Molecular Formula
C4HN2O2F3S
Molecular Weight
198.12 g/mol
Quick Actions
Basic Physical Properties
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- 5-\u200b(Trifluoromethyl)\u200b-\u200b1,\u200b3,\u200b4-\u200bthiadiazole-\u200b2-\u200bcarboxylic acid
MDL_Number
MFCD18250338
CAS Number
1260665-07-3
Recommended Suppliers
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Hubei Zhongshan Fenge Yongxue Keji Co., Ltd. China - Hubei Zhongshan Fenge Yongxue Keji Co., Ltd. |
|||||
 China - StarXin Commerce and Trade Co., Ltd. China - StarXin Commerce and Trade Co., Ltd. |
|||||
 China - Shenzhen Denolong New Materials Co., Ltd. China - Shenzhen Denolong New Materials Co., Ltd. |
|||||
 Chile - INQUISA S.A. Chile - INQUISA S.A. |
|||||
 China - Dezhou Hao Tian Group China - Dezhou Hao Tian Group |
|||||
 China - Jilin City Lin Xin Trade and Commerce Co., Ltd. China - Jilin City Lin Xin Trade and Commerce Co., Ltd. |
|||||
 Germany - URACA GmbH & Co. KG Germany - URACA GmbH & Co. KG |
|||||
 Switzerland - Bucher Industries AG Switzerland - Bucher Industries AG |
Related Compounds
Related Articles
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F


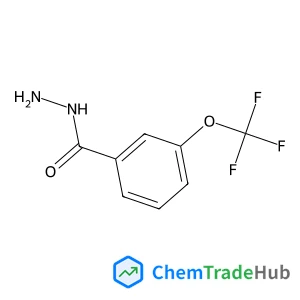
![700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline 700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline](/structs/700/700874-71-1-fbbc.webp)
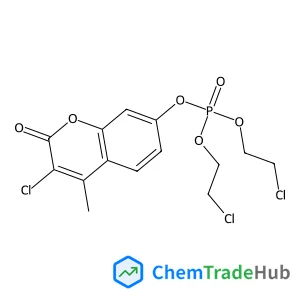
![315234-49-2 - 1-[(Tert-butoxy)carbonyl]-2-(prop-2-en-1-yl)pyrrolidine-2-carboxylic acid 315234-49-2 - 1-[(Tert-butoxy)carbonyl]-2-(prop-2-en-1-yl)pyrrolidine-2-carboxylic acid](/structs/315/315234-49-2-fe31.webp)