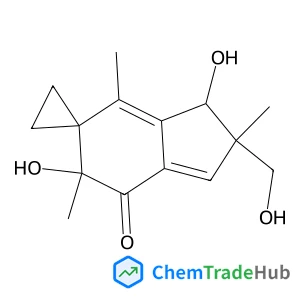
Illudin S | CAS No. 1149-99-1
Basic Information
CAS Number
1149-99-1
Molecular Formula
C15H20O4
Molecular Weight
264.32 g/mol
Quick Actions
Basic Physical Properties
Melting Point
124-126 ºC
Boiling Point
327.55°C (rough estimate)
Refractive Index
1.4300 (estimate)
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- UNII-EWJ0GY2H7X
- EWJ0GY2H7X
- (-)-Illudin S
- C09688
- SPIRO(CYCLOPROPANE-1,5'-(5H)INDEN)-7'(6'H)-ONE, 2',3'-DIHYDRO-3',6'-DIHYDROXY-2'-(HYDROXYMETHYL)-2',4',6'-TRIMETHYL-, (2'S,3'R,6'R)-
- (2'S,3'R,6'R)-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-2',3',6',7'-tetrahydrospiro[cyclopropane-1,5'-inden]-7'-one
- J-003167
- (1R,2S,5R)-1,5-dihydroxy-2-(hydroxymethyl)-2,5,7-trimethylspiro[1H-indene-6,1'-cyclopropane]-4-one
- (2'S,3'R,6'R)-2',3'-Dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethylspiro[cyclopropane-1,5'-[5H]inden]-7'(6'H)-one
- NSC 400979
- Lampterol
- NSC-626369
- (-)-1alpha,7beta,12-trihydroxy-2,9-illudadien-8-one
- Lunamycin
- CHEMBL509170
- LMPR0103590002
- NCI60_003751
- AKOS040745210
- (2'S-(2'.ALPHA.,3'.BETA.,6'.ALPHA.))-2',3'-DIHYDRO-3',6'-DIHYDROXY-2'-(HYDROXYMETHYL)-2',4',6'-TRIMETHYLSPIRO(CYCLOPROPANE-1,5'-(5H)INDEN)-7'(6'H)-ONE
- HY-125098
- AC-36761
- BRN 2336420
- Sprio(cyclopropane-1,5'-(5H)inden)-7'(6'H)-one, 2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-, (2'S,3'R,6'R)-
- SCHEMBL1743370
- CHEBI:5867
- LUNAMYCIN (OBSOLETE)
- NSC-400979
- NSC 626369
- CCRIS 3540
- EX-A5056
- 1149-99-1
- ILLUDIN S [MI]
- CS-0089316
- Spiro[cyclopropane-1, 2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-
- Q27106920
- 2',3'-Dihydro-2',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethylspiro(cyclopropane-1,5'-(5H)inden)-7'(6'H)-one
- DR-15978
- DTXSID601031301
- NSC400979
- Spiro[cyclopropane-1, 2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-, [2'S-(2'.alpha.,3'.beta.,6'.alpha.)]-
- Spiro(cyclopropane-1,5'-(5H)inden)-7'(6'H)-one, 2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-, (2'S-(2'alpha,3'beta,6'alpha))-
- Illudin S
- Illudine S
- DDLLIYKVDWPHJI-RDBSUJKOSA-N
- Spiro[cyclopropane-1,5'-[5H]inden]-7'(6'H)-one,2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-,(2'S,3'R,6'R)-
- ILLUDIN S
- LIGHT YELLOW SOLID
- Spiro[cyclopropane-1,5'-[5H]inden]-7'(6'H)-one,2',3'-dihydro-3',6'-dihydroxy-2'-(hydroxymethyl)-2',4',6'-trimethyl-,(2'S,3'R,
- (2'S)-3't,6'c-Dihydroxy-2'r-hydroxymethyl-2',4',6't-trimethyl-2',3'-dihydro-spiro[cyclopropan-1,5'-inden]-7'-on
- (2'S)-3't,6'c-dihydroxy-2'r-hydroxymethyl-2',4',6't-trimethyl-2',3'-dihydro-spiro[cyclopropane-1,5'-inden]-7'-one
- (2'S,3'R,6'R)-2',3'-Dihydro-3',6'-dihydroxy-2'-hydroxymethyl-2',4',6'-trimethylspiro(
- (2'S,3'R,6'R)-2',3'-Dihydro-3',6'-dihydroxy-2'-hydroxymethyl-2',4',6'-trimethylspiro[cyclopropane-1,5'-[5H]inden]-7'(6'H)-one
MDL_Number
MFCD00870487
CAS Number
1149-99-1
Supplier Information
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shanghai Mofu Medicine Technology Co., Ltd. China - Shanghai Mofu Medicine Technology Co., Ltd. |
|||||
 China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. China - Hubei Yangxin Pharmaceutical Technology Co., Ltd. |
|||||
 China - Shanghai Weiling BioScience Technology Co., Ltd. China - Shanghai Weiling BioScience Technology Co., Ltd. |
|||||
 China - Hubei Zhongshan Fenge Yongxue Keji Co., Ltd. China - Hubei Zhongshan Fenge Yongxue Keji Co., Ltd. |
|||||
 China - Suzhou Jinli Biotechnology Co., Ltd. China - Suzhou Jinli Biotechnology Co., Ltd. |
|||||
 China - Shanghai MinKuai Bioscience Technology Co., Ltd. China - Shanghai MinKuai Bioscience Technology Co., Ltd. |
|||||
 China - Taizhou Kede Chemical Industry Co., Ltd. China - Taizhou Kede Chemical Industry Co., Ltd. |
|||||
 China - Zhejiang Huidah Biotechnology Co., Ltd. China - Zhejiang Huidah Biotechnology Co., Ltd. |
|||||
 China - Nantong Quanyi Biotechnology Co., Ltd. China - Nantong Quanyi Biotechnology Co., Ltd. |
|||||
 China - Shanghai Aladdin Bio-Technologies Co., Ltd. China - Shanghai Aladdin Bio-Technologies Co., Ltd. |
Related Compounds
Related Articles
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
An aminophosphonate ester ligand-containing platinum(ii) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivo
Ke-Bin Huang, Feng-Yang Wang, Hai-Wen Feng, Hejiang Luo, Yan Long, Albert S. C. Chan, Rong Liu, Huahong Zou, Zhen-Feng Chen, Yan-Cheng Liu, You-Nian Liu, Hong Liang
DOI: 10.1039/C9CC06563F
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B
Interfacial engineering of a polymer–MOF composite by in situ vitrification
Rijia Lin, Jingwei Hou, Mengran Li, Zhanke Wang, Lei Ge, Shichun Li, Zhonghua Zhu, Thomas D. Bennett, Vicki Chen
DOI: 10.1039/D0CC00664E
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G

![224-53-3 - Dibenzo[c,h]acridine 224-53-3 - Dibenzo[c,h]acridine](/structs/224/224-53-3-97c9.webp)
![37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione 37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione](/structs/378/37845-14-0-f8d0.webp)

![1337532-29-2 - 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone 1337532-29-2 - 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone](/structs/133/1337532-29-2-edb6.webp)
![24449-39-6 - 2,2,2',2'-Tetramethyl-2H,2'H-5,5'-bibenzo[h]chromene-6,6'-diol 24449-39-6 - 2,2,2',2'-Tetramethyl-2H,2'H-5,5'-bibenzo[h]chromene-6,6'-diol](/structs/244/24449-39-6-3118.webp)